Abstract
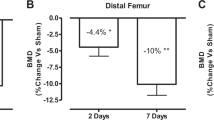
To elucidate mechanisms of bone loss after spinal cord injury (SCI), we evaluated the time-course of cancellous and cortical bone microarchitectural deterioration via microcomputed tomography, measured histomorphometric and circulating bone turnover indices, and characterized the development of whole bone mechanical deficits in a clinically relevant experimental SCI model. 16-weeks-old male Sprague–Dawley rats received T9 laminectomy (SHAM, n = 50) or moderate–severe contusion SCI (n = 52). Outcomes were assessed at 2-weeks, 1-month, 2-months, and 3-months post-surgery. SCI produced immediate sublesional paralysis and persistent hindlimb locomotor impairment. Higher circulating tartrate-resistant acid phosphatase 5b (bone resorption marker) and lower osteoblast bone surface and histomorphometric cancellous bone formation indices were present in SCI animals at 2-weeks post-surgery, suggesting uncoupled cancellous bone turnover. Distal femoral and proximal tibial cancellous bone volume, trabecular thickness, and trabecular number were markedly lower after SCI, with the residual cancellous network exhibiting less trabecular connectivity. Periosteal bone formation indices were lower at 2-weeks and 1-month post-SCI, preceding femoral cortical bone loss and the development of bone mechanical deficits at the distal femur and femoral diaphysis. SCI animals also exhibited lower serum testosterone than SHAM, until 2-months post-surgery, and lower serum leptin throughout. Our moderate–severe contusion SCI model displayed rapid cancellous bone deterioration and more gradual cortical bone loss and development of whole bone mechanical deficits, which likely resulted from a temporal uncoupling of bone turnover, similar to the sequalae observed in the motor-complete SCI population. Low testosterone and/or leptin may contribute to the molecular mechanisms underlying bone deterioration after SCI.






Similar content being viewed by others
References
Bauman WA, Cardozo CP (2015) Osteoporosis in individuals with spinal cord injury. PMR 7(2):188–201
Dauty M, Perrouin Verbe B, Maugars Y, Dubois C, Mathe JF (2000) Supralesional and sublesional bone mineral density in spinal cord-injured patients. Bone 27(2):305–309
Eser P, Frotzler A, Zehnder Y, Wick L, Knecht H, Denoth J, Schiessl H (2004) Relationship between the duration of paralysis and bone structure: a pQCT study of spinal cord injured individuals. Bone 34(5):869–880
Frotzler A, Berger M, Knecht H, Eser P (2008) Bone steady-state is established at reduced bone strength after spinal cord injury: a longitudinal study using peripheral quantitative computed tomography (pQCT). Bone 43(3):549–555
Frisbie JH (1997) Fractures after myelopathy: the risk quantified. J Spinal Cord Med 20(1):66–69
Morse LR, Battaglino RA, Stolzmann KL, Hallett LD, Waddimba A, Gagnon D, Lazzari AA, Garshick E (2009) Osteoporotic fractures and hospitalization risk in chronic spinal cord injury. Osteoporos Int 20(3):385–392
Grassner L, Klein B, Maier D, Buhren V, Vogel M (2017) Lower extremity fractures in patients with spinal cord injury characteristics, outcome and risk factors for non-unions. J Spinal Cord Med. https://doi.org/10.1080/10790268.2017.1329915
Jiang SD, Jiang LS, Dai LY (2007) Changes in bone mass, bone structure, bone biomechanical properties, and bone metabolism after spinal cord injury: a 6-month longitudinal study in growing rats. Calcif Tissue Int 80(3):167–175
Sharif-Alhoseini M, Khormali M, Rezaei M et al (2017) Animal models of spinal cord injury: a systematic review. Spinal Cord 55(8):714–721
Morse L, Teng YD, Pham L et al (2008) Spinal cord injury causes rapid osteoclastic resorption and growth plate abnormalities in growing rats (SCI-induced bone loss in growing rats). Osteoporos Int 19(5):645–652
Morse LR, Xu Y, Solomon B, Boyle L, Yoganathan S, Stashenko P, Battaglino RA (2011) Severe spinal cord injury causes immediate multi-cellular dysfunction at the chondro-osseous junction. Transl Stroke Res 2(4):643–650
Yarrow JF, Ye F, Balaez A et al (2014) Bone loss in a new rodent model combining spinal cord injury and cast immobilization. J Musculoskelet Neuronal Interact 14(3):255–266
Voor MJ, Brown EH, Xu Q, Waddell SW, Burden RL Jr, Burke DA, Magnuson DS (2012) Bone loss following spinal cord injury in a rat model. J Neurotrauma 29(8):1676–1682
Lin T, Tong W, Chandra A et al (2015) A comprehensive study of long-term skeletal changes after spinal cord injury in adult rats. Bone Res 3:15028
Lin CY, Androjna C, Rozic R, Nguyen BT, Parsons B, Midura RJ, Lee YS (2018) Differential adaptations of the musculoskeletal system following spinal cord contusion and transection in rats. J Neurotrauma 35(15):1737–1744
Jiang SD, Jiang LS, Dai LY (2006) Spinal cord injury causes more damage to bone mass, bone structure, biomechanical properties and bone metabolism than sciatic neurectomy in young rats. Osteoporos Int 17(10):1552–1561
Liu D, Zhao CQ, Li H, Jiang SD, Jiang LS, Dai LY (2008) Effects of spinal cord injury and hindlimb immobilization on sublesional and supralesional bones in young growing rats. Bone 43(1):119–125
Jiang SD, Shen C, Jiang LS, Dai LY (2007) Differences of bone mass and bone structure in osteopenic rat models caused by spinal cord injury and ovariectomy. Osteoporos Int 18(6):743–750
Devivo MJ (2012) Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 50(5):365–372
Basso DM, Beattie MS, Bresnahan JC (1996) Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transection. Exp Neurol 139(2):244–256
Yarrow JF, Conover CF, Beggs LA et al (2014) Testosterone dose dependently prevents bone and muscle loss in rodents after spinal cord injury. J Neurotrauma 31(9):834–845
Yarrow JF, Phillips EG, Conover CF et al (2017) Testosterone plus finasteride prevents bone loss without prostate growth in a rodent spinal cord injury model. J Neurotrauma 34(21):2972–2981
Beggs LA, Ye F, Ghosh P et al (2015) Sclerostin inhibition prevents spinal cord injury-induced cancellous bone loss. J Bone Miner Res 30(4):681–689
Yarrow JF, Conover CF, Purandare AV, Bhakta AM, Zheng N, Conrad B, Altman MK, Franz SE, Wronski TJ, Borst SE (2008) Supraphysiological testosterone enanthate administration prevents bone loss and augments bone strength in gonadectomized male and female rats. Am J Physiol Endocrinol Metab 295(5):E1213–E1222
McCoy SC, Yarrow JF, Conover CF et al (2012) 17beta-Hydroxyestra-4,9,11-trien-3-one (Trenbolone) preserves bone mineral density in skeletally mature orchiectomized rats without prostate enlargement. Bone 51(4):667–673
Carbone LD, Chin AS, Burns SP, Svircev JN, Hoenig H, Heggeness M, Bailey L, Weaver F (2014) Mortality after lower extremity fractures in men with spinal cord injury. J Bone Miner Res 29(2):432–439
Reiter AL, Volk A, Vollmar J, Fromm B, Gerner HJ (2007) Changes of basic bone turnover parameters in short-term and long-term patients with spinal cord injury. Eur Spine J 16(6):771–776
Jiang SD, Yan J, Jiang LS, Dai LY (2011) Down-regulation of the Wnt, estrogen receptor, insulin-like growth factor-I, and bone morphogenetic protein pathways in osteoblasts from rats with chronic spinal cord injury. Joint Bone Spine 78(5):488–492
Zhao W, Li X, Peng Y et al. (2018) Sclerostin antibody reverses the severe sublesional bone loss in rats after chronic spinal cord injury. Calcif Tissue Int. https://doi.org/10.1007/s00223-018-0439-8
Modrowski D, del Pozo E, Miravet L (1992) Dynamics of circulating osteocalcin in rats during growth and under experimental conditions. Horm Metab Res 24(10):474–477
Han B, Copeland M, Geiser AG, Hale LV, Harvey A, Ma YL, Powers CS, Sato M, You J, Hale JE (2007) Development of a highly sensitive, high-throughput, mass spectrometry-based assay for rat procollagen type-I N-terminal propeptide (PINP) to measure bone formation activity. J Proteome Res 6(11):4218–4229
Ito M, Nishida A, Koga A, Ikeda S, Shiraishi A, Uetani M, Hayashi K, Nakamura T (2002) Contribution of trabecular and cortical components to the mechanical properties of bone and their regulating parameters. Bone 31(3):351–358
Bauman WA, La Fountaine MF, Cirnigliaro CM, Kirshblum SC, Spungen AM (2017) Testicular responses to hCG stimulation at varying doses in men with spinal cord injury. Spinal Cord 55(7):659–663
Sullivan SD, Nash MS, Tefera E, Tinsley E, Blackman MR, Groah S (2017) Prevalence and etiology of hypogonadism in young men with chronic spinal cord injury: a cross-sectional analysis from two university-based rehabilitation centers. PMR 9(8):751–760
Maimoun L, Lumbroso S, Paris F, Couret I, Peruchon E, Rouays-Mabit E, Rossi M, Leroux JL, Sultan C (2006) The role of androgens or growth factors in the bone resorption process in recent spinal cord injured patients: a cross-sectional study. Spinal Cord 44(12):791–797
Park AJ, Battaglino RA, Nguyen NMH, Morse LR (2018) Associations between lean mass and leptin in men with chronic spinal cord injury: results from the FRASCI-muscle study. PLoS ONE 13(6):e0198969
Phillips EG, Beggs LA, Ye F, Conover CF, Beck DT, Otzel DM, Ghosh P, Bassit ACF, Borst SE, Yarrow JF (2018) Effects of pharmacologic sclerostin inhibition or testosterone administration on soleus muscle atrophy in rodents after spinal cord injury. PLoS ONE 13(3):e0194440
Powell D, Affuso O, Chen Y (2017) Weight change after spinal cord injury. J Spinal Cord Med 40(2):130–137
Doubelt I, Totosy de Zepetnek J, MacDonald MJ, Atkinson SA (2015) Influences of nutrition and adiposity on bone mineral density in individuals with chronic spinal cord injury: A cross-sectional, observational study. Bone Rep 2:26–31
Sabour H, Norouzi Javidan A, Latifi S, Shidfar F, Vafa MR, Emami Razavi SH, Larijani B, Heshmat R (2015) Relationship between leptin and adiponectin concentrations in plasma and femoral and spinal bone mineral density in spinal cord-injured individuals. Spine J 15(1):1–9
Turner RT, Kalra SP, Wong CP, Philbrick KA, Lindenmaier LB, Boghossian S, Iwaniec UT (2013) Peripheral leptin regulates bone formation. J Bone Miner Res 28(1):22–34
Baek K, Bloomfield SA (2009) Beta-adrenergic blockade and leptin replacement effectively mitigate disuse bone loss. J Bone Miner Res 24(5):792–799
Qin W, Li X, Peng Y et al (2016) Sclerostin antibody preserves the morphology and structure of osteocytes and blocks the severe skeletal deterioration after motor-complete spinal cord injury in rats. J Bone Miner Res 31(7):1482
Gifre L, Vidal J, Carrasco JL, Filella X, Ruiz-Gaspa S, Muxi A, Portell E, Monegal A, Guanabens N, Peris P (2015) Effect of recent spinal cord injury on wnt signaling antagonists (sclerostin and dkk-1) and their relationship with bone loss. A 12-month prospective study. J Bone Miner Res 30(6):1014–1021
Qin W, Li X, Peng Y et al (2015) Sclerostin antibody preserves the morphology and structure of osteocytes and blocks the severe skeletal deterioration after motor-complete spinal cord injury in rats. J Bone Miner Res 30(11):1994–2004
Pruss H, Tedeschi A, Thiriot A et al (2017) Spinal cord injury-induced immunodeficiency is mediated by a sympathetic-neuroendocrine adrenal reflex. Nat Neurosci 20(11):1549–1559
Zhang Y, Guan Z, Reader B et al (2013) Autonomic dysreflexia causes chronic immune suppression after spinal cord injury. J Neurosci 33(32):12970–12981
Samantaray S, Das A, Matzelle DC, Yu SP, Wei L, Varma A, Ray SK, Banik NL (2016) Administration of low dose estrogen attenuates persistent inflammation, promotes angiogenesis, and improves locomotor function following chronic spinal cord injury in rats. J Neurochem 137(4):604–617
Yarrow JF, McCoy SC, Ferreira JA, Pingel JE, Conrad BP, Wronski TJ, Williams AA, Borst SE, Brown M (2012) A rehabilitation exercise program induces severe bone mineral deficits in estrogen-deficient rats after extended disuse. Menopause 19(11):1267–1276
Yarrow JF, Wronski TJ, Borst SE (2015) Testosterone and adult male bone: actions independent of 5 alpha-reductase and aromatase. Exerc Sport Sci Rev 43(4):222–230
Acknowledgements
This work was supported by the Office of Research and Development, Rehabilitation Research and Development (RR&D) Service, Department of Veterans Affairs (SPiRE 1I21RX001273-01 and PECASE #B9280-O) to JFY, and by resources provided by the North Florida/South Georgia Veterans Health System. The work reported herein does not represent the views of the US Department of Veterans Affairs or the US Government.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Dana M. Otzel, Christine F. Conover, Fan Ye, Ean G. Phillips, Taylor Bassett, Russell D. Wnek, Micah Flores, Andrea Catter, Payal Ghosh, Alexander Balaez, Jason Petusevsky, Cong Chen, Yongxin Gao, Yi Zhang, Jessica M. Jiron, Prodip K. Bose, Stephen E. Borst, Thomas J. Wronski, J. Ignacio Aguirre and Joshua F. Yarrow declares that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All experimental procedures conformed to the ILAR Guide to the Care and Use of Experimental Animals and were approved by the Institutional Animal Care and Use Committee at the Malcom Randall VA Medical Center.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Otzel, D.M., Conover, C.F., Ye, F. et al. Longitudinal Examination of Bone Loss in Male Rats After Moderate–Severe Contusion Spinal Cord Injury. Calcif Tissue Int 104, 79–91 (2019). https://doi.org/10.1007/s00223-018-0471-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-0471-8