Abstract
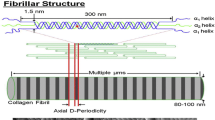
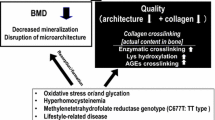
Collagen cross-linking, as a form of collagen post-translational modification, plays a crucial role in maintaining bone mechanical properties as well as in regulating cell biological functions. Shifts in cross-links profile are found apparently correlated to kinds of skeletal pathology and diseases, whereas little is known about the relationship between collagen cross-links and osteogenesis. Here, we hypothesized that the inhibition of collagen cross-links could impair skeletal microstructure and inhibit osteogenesis. A mouse model of collagen cross-linking defects has been established using subcutaneous injection of 350 mg/kg β-aminopropionitrile (BAPN) daily for 4 weeks, and same dose of phosphate buffered saline (PBS) served as control group. The analysis of bone microstructural parameters revealed a significant decrease of bone volume fraction (BV/TV) and trabecular thickness (Tb.Th), and increase of bone surface ratio (BS/BV), structure model index (SMI) as well as trabecular separation (Tb.Sp) in the experimental group (p < 0.05), whereas there was no difference observed in bone mineral density (BMD). Histological staining displayed that the BAPN treatment caused thinner trabeculae and decrease of collagen content in proximal tibiae. The analysis of osteogenesis PCR (Polymerase Chain Reaction) array reflected that BAPN remarkably influenced the expression of Alpl, Bglap, Bgn, Bmp5, Col10a1, Col1a1, Col1a2, Col5a1, Itga2b, and Serpinh1. The results of immunohistochemistry displayed a significant reduction in the mean optical densities of OCN and COL1 at the presence of BAPN. The overall results of this study suggested that BAPN alters bone microstructure and hinders the expression of osteogenic genes without affecting mineralization processes, indicating the influences of collagen cross-links on osteogenesis may be a potential pathological mechanism in skeletal diseases.





Similar content being viewed by others
References
Saito M, Marumo K (2015) Effects of collagen crosslinking on bone material properties in health and disease. Calcif Tissue Int 97:242–261. https://doi.org/10.1007/s00223-015-9985-5
Yamauchi M, Sricholpech M (2012) Lysine post-translational modifications of collagen. Essays Biochem 52:113–133. https://doi.org/10.1042/bse0520113
Smith-Mungo LI, Kagan HM (1998) Lysyl oxidase: properties, regulation and multiple functions in biology. Matrix Biol 16:387–398
Kagan HM, Trackman PC (1991) Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol 5:206–210. https://doi.org/10.1165/ajrcmb/5.3.206
McNerny EMB, Gong B, Morris MD, Kohn DH (2015) Bone fracture toughness and strength correlate with collagen cross-link maturity in a dose-controlled lathyrism mouse model. J Bone Miner Res 30:455–464. https://doi.org/10.1002/jbmr.2356
Oxlund H, Barckman M, Ørtoft G, Andreassen TT (1995) Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone 17:365S–371S
Rouby El DH, Bashir MH, Korany NS (2008) Ultrastructural and histomorphometric alterations of rat jaw bones after experimental induction of lathyrism. Arch Oral Biol 53:916–923. https://doi.org/10.1016/j.archoralbio.2008.04.008
Banse X, Sims TJ, Bailey AJ (2002) Mechanical properties of adult vertebral cancellous bone: correlation with collagen intermolecular cross-links. J Bone Miner Res 17:1621–1628. https://doi.org/10.1359/jbmr.2002.17.9.1621
Viguet-Carrin S, Garnero P, Delmas PD (2005) The role of collagen in bone strength. Osteoporos Int 17:319–336. https://doi.org/10.1007/s00198-005-2035-9
Reichenberger E, Olsen BR (1996) Collagens as organizers of extracellular matrix during morphogenesis. Semin Cell Dev Biol 7:631–638. https://doi.org/10.1006/scdb.1996.0077
Paschalis EP, Tatakis DN, Robins S et al (2011) Lathyrism-induced alterations in collagen cross-links influence the mechanical properties of bone material without affecting the mineral. Bone 49:1232–1241. https://doi.org/10.1016/j.bone.2011.08.027
Garnero P, Borel O, Gineyts E et al (2006) Extracellular post-translational modifications of collagen are major determinants of biomechanical properties of fetal bovine cortical bone. Bone 38:300–309. https://doi.org/10.1016/j.bone.2005.09.014
Trappmann B (2012) Extracellular-matrix tethering regulates stem-cell fate. Nat Mater 11:642–649. https://doi.org/10.1038/nmat3339
Engler AJ, Sen S, Sweeney HL, Discher DE (2006) Matrix elasticity directs stem cell lineage specification. Cell 126:677–689. https://doi.org/10.1016/j.cell.2006.06.044
Shih Y-RV, Tseng K-F, Lai H-Y et al (2011) Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. J Bone Miner Res 26:730–738. https://doi.org/10.1002/jbmr.278
Bank RA, Robins SP, Wijmenga C et al (1999) Defective collagen crosslinking in bone, but not in ligament or cartilage, in Bruck syndrome: indications for a bone-specific telopeptide lysyl hydroxylase on chromosome 17. Proc Natl Acad Sci USA 96:1054–1058
Bank RA, Tekoppele JM, Janus GJ et al (2000) Pyridinium cross-links in bone of patients with osteogenesis imperfecta: evidence of a normal intrafibrillar collagen packing. J Bone Miner Res 15:1330–1336. https://doi.org/10.1359/jbmr.2000.15.7.1330
Forlino A, Cabral WA, Barnes AM, Marini JC (2011) New perspectives on osteogenesis imperfecta. Nat Rev Endocrinol 7:540–557. https://doi.org/10.1038/nrendo.2011.81
Paschalis EP, Gamsjaeger S, Fratzl-Zelman N et al (2016) Evidence for a role for nanoporosity and pyridinoline content in human mild osteogenesis imperfecta. J Bone Miner Res 31:1050–1059. https://doi.org/10.1002/jbmr.2780
Saito M, Marumo K (2009) Collagen cross-links as a determinant of bone quality: a possible explanation for bone fragility in aging, osteoporosis, and diabetes mellitus. Osteoporos Int 21:195–214. https://doi.org/10.1007/s00198-009-1066-z
Oxlund H, Mosekilde L, Ørtoft G (1996) Reduced concentration of collagen reducible cross links in human trabecular bone with respect to age and osteoporosis. Bone 19:479–484
Canelón SP, Wallace JM (2016) β-aminopropionitrile-induced reduction in enzymatic crosslinking causes in vitro changes in collagen morphology and molecular composition. PLoS ONE 11:e0166392–e0166313. https://doi.org/10.1371/journal.pone.0166392
Bouxsein ML, Boyd SK, Christiansen BA et al (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25:1468–1486. https://doi.org/10.1002/jbmr.141
Baron R (1989) Molecular mechanisms of bone resorption by the osteoclast. Anat Rec 224:317–324. https://doi.org/10.1002/ar.1092240220
Morinobu M, Nakamoto T, Hino K et al (2005) The nucleocytoplasmic shuttling protein CIZ reduces adult bone mass by inhibiting bone morphogenetic protein-induced bone formation. J Exp Med 201:961–970. https://doi.org/10.1084/jem.20041097
Oxlund H, Barckman M, Ørtoft G, Andreassen TT (1995) Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone 17:S365–S371. https://doi.org/10.1016/8756-3282(95)00328-B
Mizoguchi F, Izu Y, Hayata T et al (2010) Osteoclast-specific Dicer gene deficiency suppresses osteoclastic bone resorption. J Cell Biochem 109:866–875. https://doi.org/10.1002/jcb.22228
Matsuzawa T, Anderson HC (1971) Phosphatases of epiphyseal cartilage studied by electron microscopic cytochemical methods. J Histochem Cytochem 19:801–808. https://doi.org/10.1177/19.12.801
Eliades A, Papadantonakis N, Bhupatiraju A et al (2011) Control of megakaryocyte expansion and bone marrow fibrosis by lysyl oxidase. J Biol Chem 286:27630–27638. https://doi.org/10.1074/jbc.M111.243113
Fernandes H, Dechering K, Van Someren E et al (2009) The role of collagen crosslinking in differentiation of human mesenchymal stem cells and MC3T3-E1 cells. Tissue Eng A 15:3857–3867. https://doi.org/10.1089/ten.tea.2009.0011
Morgan SL, Prater GL (2017) Quality in dual-energy X-ray absorptiometry scans. Bone. https://doi.org/10.1016/j.bone.2017.01.033
Shi J, Lee S, Uyeda M et al (2016) Guidelines for dual energy X-ray absorptiometry analysis of trabecular bone-rich regions in mice: improved precision, accuracy, and sensitivity for assessing longitudinal bone changes. Tissue Eng C 22:451–463. https://doi.org/10.1089/ten.tec.2015.0383
Kahai S, Vary CPH, Gao Y, Seth A (2004) Collagen, type V, alpha1 (COL5A1) is regulated by TGF-beta in osteoblasts. Matrix Biol 23:445–455. https://doi.org/10.1016/j.matbio.2004.09.004
Kingsley DM, Bland AE, Grubber JM et al (1992) The mouse short ear skeletal morphogenesis locus is associated with defects in a bone morphogenetic member of the TGF beta superfamily. Cell 71:399–410
Xu T, Bianco P, Fisher LW et al (1998) Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet 20:78–82. https://doi.org/10.1038/1746
Coleman R, Brown J, Terpos E et al (2008) Bone markers and their prognostic value in metastatic bone disease: clinical evidence and future directions. Cancer Treat Rev 34:629–639. https://doi.org/10.1016/j.ctrv.2008.05.001
Anderson HC, Sipe JB, Hessle L et al (2004) Impaired calcification around matrix vesicles of growth plate and bone in alkaline phosphatase-deficient mice. Am J Pathol 164:841–847
Seibel MJ (2005) Biochemical markers of bone turnover: part I: biochemistry and variability. Clin Biochem Rev 26:97–122
Neve A, Corrado A, Cantatore FP (2013) Osteocalcin: skeletal and extra-skeletal effects. J Cell Physiol 228:1149–1153. https://doi.org/10.1002/jcp.24278
Turecek C, Fratzl-Zelman N, Rumpler M et al (2008) Collagen cross-linking influences osteoblastic differentiation. Calcif Tissue Int 82:392–400. https://doi.org/10.1007/s00223-008-9136-3
Parisuthiman D, Mochida Y, Duarte WR, Yamauchi M (2005) Biglycan modulates osteoblast differentiation and matrix mineralization. J Bone Miner Res 20:1878–1886. https://doi.org/10.1359/JBMR.050612
Vijayan V, Gupta S, Gupta S (2017) Bone morphogenetic protein-5, a key molecule that mediates differentiation in MC3T3E1 osteoblast cell line. Biofactors 20:343. https://doi.org/10.1002/biof.1360
Ho AM, Marker PC, Peng H et al (2008) Dominant negative Bmp5 mutation reveals key role of BMPs in skeletal response to mechanical stimulation. BMC Dev Biol 8:35. https://doi.org/10.1186/1471-213X-8-35
Mitjavila-Garcia MT, Cailleret M, Godin I et al (2002) Expression of CD41 on hematopoietic progenitors derived from embryonic hematopoietic cells. Development 129:2003–2013
Farjanel J, Sève S, Borel A et al (2005) Inhibition of lysyl oxidase activity can delay phenotypic modulation of chondrocytes in two-dimensional culture. Osteoarthr Cartil 13:120–128. https://doi.org/10.1016/j.joca.2004.06.015
Warman ML, Abbott M, Apte SS et al (1993) A type X collagen mutation causes Schmid metaphyseal chondrodysplasia. Nat Genet 5:79–82. https://doi.org/10.1038/ng0993-79
Jacenko O, LuValle PA, Olsen BR (1993) Spondylometaphyseal dysplasia in mice carrying a dominant negative mutation in a matrix protein specific for cartilage-to-bone transition. Nature 365:56–61. https://doi.org/10.1038/365056a0
Nagai N, Hosokawa M, Itohara S et al (2000) Embryonic lethality of molecular chaperone Hsp47 knockout mice is associated with defects in collagen biosynthesis. J Cell Biol 150:1499–1506. https://doi.org/10.1083/jcb.150.6.1499
Masago Y, Hosoya A, Kawasaki K et al (2012) The molecular chaperone Hsp47 is essential for cartilage and endochondral bone formation. J Cell Sci 125:1118–1128. https://doi.org/10.1242/jcs.089748
Väänänen K, Morris DC, Munoz PA, Parvinen EK (1987) Immunohistochemical study of alkaline phosphatase in growth plate cartilage, bone, and fetal calf isolated chondrocytes using monoclonal antibodies. Acta Histochem 82:211–217. https://doi.org/10.1016/S0065-1281(87)80032-6
Augello A, De Bari C (2010) The regulation of differentiation in mesenchymal stem cells. Hum Gene Ther 21:1226–1238. https://doi.org/10.1089/hum.2010.173
Discher DE, Mooney DJ, Zandstra PW (2009) Growth factors, matrices, and forces combine and control stem cells. Science 324:1673–1677. https://doi.org/10.1126/science.1171643
Harris GM, Piroli ME, Jabbarzadeh E (2013) Deconstructing the effects of matrix elasticity and geometry in mesenchymal stem cell lineage commitment. Adv Funct Mater 24:2396–2403. https://doi.org/10.1002/adfm.201303400
Acknowledgements
This work was supported by the National Nature Science Foundation of China (Grant No. 31470904).
Author information
Authors and Affiliations
Contributions
Study design: Yu Shen, Pu Yang, and Jin Hao. Study conduct: Yu Shen, Dian Jing, and Ge Tang. Data collection: Yu Shen and Dian Jing. Data analysis: Yu Shen, Pu Yang, and Dian Jing. Data interpretation: Yu Shen, Pu Yang, and Dian Jing. Drafting manuscript: Yu Shen. Revising manuscript content: Yu Shen, Pu Yang, and Zhihe Zhao. Approving final version of manuscript: Yu Shen, Pu Yang, Dian Jing, Jin Hao, Ge Tang, and Zhihe Zhao. Yu Shen and Zhihe Zhao take responsibility for the integrity of the data analysis.
Corresponding authors
Ethics declarations
Conflict of interest
Yu Shen, Dian Jing, Jin Hao, Ge Tang, Pu Yang, and Zhihe Zhao declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
All the treatments described in this research were reviewed and approved by the Ethics Committee of State Key Laboratory of Oral Diseases at the Sichuan University.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Shen, Y., Jing, D., Hao, J. et al. The Effect of β-Aminopropionitrile on Skeletal Micromorphology and Osteogenesis. Calcif Tissue Int 103, 411–421 (2018). https://doi.org/10.1007/s00223-018-0430-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-0430-4