Abstract
Alkaline phosphatases (APs) remove the phosphate (dephosphorylation) needed in multiple metabolic processes (from many molecules such as proteins, nucleotides, or pyrophosphate). Therefore, APs are important for bone mineralization but paradoxically they can also be deleterious for other processes, such as vascular calcification and the increasingly known cross-talk between bone and vessels. A proper balance between beneficial and harmful activities is further complicated in the context of chronic kidney disease (CKD). In this narrative review, we will briefly update the complexity of the enzyme, including its different isoforms such as the bone-specific alkaline phosphatase or the most recently discovered B1x. We will also analyze the correlations and potential discrepancies with parathyroid hormone and bone turnover and, most importantly, the valuable recent associations of AP’s with cardiovascular disease and/or vascular calcification, and survival. Finally, a basic knowledge of the synthetic and degradation pathways of APs promises to open new therapeutic strategies for the treatment of the CKD-Mineral and Bone Disorder (CKD-MBD) in the near future, as well as for other processes such as sepsis, acute kidney injury, inflammation, endothelial dysfunction, metabolic syndrome or, in diabetes, cardiovascular complications. However, no studies have been done using APs as a primary therapeutic target for clinical outcomes, and therefore, AP’s levels cannot yet be used alone as an isolated primary target in the treatment of CKD-MBD. Nonetheless, its diagnostic and prognostic potential should be underlined.




Similar content being viewed by others
References
Mazzaferro S, Tartaglione L, Rotondi S, Bover J, Goldsmith D, Pasquali M (2014) News on biomarkers in CKD-MBD. Semin Nephrol 34(6):598–611
Lomashvili K, Garg P, Narisawa S, Millan JL, O’neill WC (2008) Upregulation of alkaline phosphatase and pyrophosphate hydrolysis: potential mechanism for uremic vascular calcification. Kidney Int 73(9):1024–1030
Schoppet M, Shanahan CM (2008) Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int 73(9):989–991
Schibler D, Russell RGG, Fleisch H (1968) Inhibition by pyrophosphate and poly-phosphate of aortic calcification induced by vitamin D3 in rats. Clin Sci 35:363–372
London GM (2012) Bone-vascular cross-talk. J Nephrol 25(5):619–625
Bover J, Ureña-Torres P, Lloret MJ, Ruiz-García C, DaSilva I, Diaz-Encarnacion M, Mercado C, Mateu S, Fernandez E, Ballarin J (2016) Integral pharmacological management of bone mineral disorders in chronic kidney disease (part I): from treatment of phosphate imbalance to control of PTH and prevention of progression of cardiovascular calcification. Expert Opin Pharmacother 17(9):1247–1258
Bover J, Ureña-Torres P, Lloret MJ, Ruiz C, DaSilva I, Diaz-Encarnacion MM, Mercado C, Mateu S, Fernández E, Ballarin J (2016) Integral pharmacological management of bone mineral disorders in chronic kidney disease (part II): from treatment of phosphate imbalance to control of PTH and prevention of progression of cardiovascular calcification. Expert Opin Pharmacother 17(10):1363–1373
Shantouf R, Kovesdy CP, Kim Y, Ahmadi N, Luna A, Luna C, Rambod M, Nissenson AR, Budoff MJ, Kalantar-Zadeh K (2009) Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol 4(6):1106–1114
Lau W, Kalantar-Zadeh K (2014) Towards the revival of alkaline phosphatase for the management of bone disease, mortality and hip fractures. Nephrol Dial Transplant 29:1450–1452
Harris H (1990) The human alkaline phosphatases: what we know and what we don’t know. Clin Chim Acta 186(2):133–150
Linder C, Narisawa S, Millán L, Magnusson P (2009) Glycosylation differences contribute to distinct catalytic properties among bone alkaline phosphatase isoforms. Bone 45(5):987–993
Bervoets R, Spasovski B, Behets J, Dams G, Polenakovic H, Zafirovska K, D’Haese, C (2003) Useful biochemical markers for diagnosing renal osteodystrophy in predialysis end-stage renal failure patients. Am J Kidney Dis 41(5):997–1007
Coen G, Ballanti P, Bonucci E, Calabria S, Centorrino M, Fassino V, Sardella D (1998) Bone markers in the diagnosis of low turnover osteodystrophy in haemodialysis patients. Nephrol Dial Transplant 13(9):2294–2302
Ortega O, Rodriguez I, Hinostroza J, Laso N, Callejas R, Gallar P, Vigil A (2011) Serum alkaline phosphatase levels and left ventricular diastolic dysfunction in patients with advanced chronic kidney disease. Nephron Extra 1(1):283–291
Walker AW (1974) Increased intestinal alkaline phosphatase in serum of patients on maintenance haemodialysis. Lancet 303(7862):866–867
De Broe E, Van Hoof O (1991) Multiple forms of alkaline phosphatase in plasma of hemodialysis patients. Clin Chem 37(6):783–784
Tibi L, Chabra C, Sweeting M, Winney J, Smith F (1991) Multiple forms of alkaline phosphatase in plasma of hemodialysis patients. Clin Chem 37(6):815–820
Zetterberg H (2005) Increased serum concentrations of intestinal alkaline phosphatase in peritoneal dialysis. Clin Chem 51(3):675–676
Haarhaus M, Brandenburg V, Kalantar-Zadeh K, Stenvinkel P, Magnusson P (2017) Alkaline phosphatase: a novel treatment target for cardiovascular disease in CKD. Nature Rev Nephrol 13(7):429–442
Ureña Torres P, de Vernejoul C (1999) Circulating biochemical markers of bone remodeling in uremic patients. Kidney Int 55(6):2141–2156
Ureña Torres P, Hruby M, Ferreira A, Ang KS, de Vernejoul MC (1996) Plasma total versus bone alkaline phosphatase as markers of bone turnover in hemodialysis patients. J Am Soc Nephrol 7(3):506–512
Couttenye M, D’Haese C, Van Hoof O, Lemoniatou E, Goodman W, Verpooten A, De Broe E (1996) Low serum levels of alkaline phosphatase of bone origin: a good marker of adynamic bone disease in haemodialysis patients. Nephrol Dial Transplant 11(6):1065–1072
Behets J, Spasovski G, Sterling R, Goodman G, Spiegel M, De Broe E, D’haese PC (2015) Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int 87(4):846–856
Sprague SM, Bellorin-Font E, Jorgetti V, Carvalho AB, Malluche HH, Ferreira A, Rojas E (2016) Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am J Kidney Dis 67(4):559–566
Ueda M, Inaba M, Okuno S, Maeno Y, Ishimura E, Yamakawa T, Nishizawa Y (2005) Serum BAP as the clinically useful marker for predicting BMD reduction in diabetic hemodialysis patients with low PTH. Life Sci 77(10):1130–1139
Bover J, Jara A, Trinidad P, Rodriguez M, Martin-Malo A, Felsenfeld AJ (1994) The calcemic response to PTH in the rat: effect of elevated PTH levels and uremia. Kidney Int 46(2):310–317
Bover J, Jara A, Trinidad P, Rodriguez M, Felsenfeld A (1999) Dynamics of skeletal resistance to parathyroid hormone in the rat: effect of renal failure and dietary phosphorus. Bone 25(3):279 – 85
Delanaye P, Dubois E, Jouret F, Krzesinski M, Moranne O, Cavalier E (2013) Parathormone and bone-specific alkaline phosphatase for the follow-up of bone turnover in hemodialysis patients: is it so simple? Clin Chim Acta 417:35–38
Llach F, Bover J (2000). Renal Osteodystrophies. Brenner BM, editor. Brenner and Rector´s “The Kidney”. 6th edn. Filadelfia. W.B. Saunders Company. pp 2103–2186
Evenepoel P, Bover J, Ureña Torres P (2016) Parathyroid hormone metabolism and signaling in health and chronic kidney disease. Kidney Int 90(6):1184–1190
Evanson JM (1966) The response to the infusion of parathyroid extract in hypocalcaemic states. Clin Sci 31(1):63–75
Massry S, Stein R, Garty J, Arieff A, Coburn J, Norman A, Friedler R (1976) Skeletal resistance to the calcemic action of parathyroid hormone in uremia: role of 1,25 (OH)2 D3. Kidney Int 9(6):467–474
Llach F, Massry S, Singer F, Kurokawa K, Kaye J, Coburn J (1975, August) Skeletal resistance to endogenous parathyroid hormone in patients with early renal failure. A possible cause for secondary hyperparathyroidism. J Clin Endocrinol Metab 41(2):339–345
Wilson L, Felsenfeld A, Drezner MK, Llach F (1985). Altered divalent ion metabolism in early renal failure: role of 1,25(OH)2D. Kidney Int 27(3):565–573
Bover J, Rodriguez M, Trinidad P, Jara A, Martinez ME, Machado L, Llach F, Felsenfeld AJ (1994) Factors in the development of secondary hyperparathyroidism during graded renal failure in the rat. Kidney Int 1994;45(4):953 – 61
Andress L, Howard A, Birnbaum S (1991) Identification of a low molecular weight inhibitor of osteoblast mitogenesis in uremic plasma. Kidney Int 39(5):942–945
Mathew S, Davies M, Lund R, Saab G, Hruska A (2006) Function and effect of bone morphogenetic protein-7 in kidney bone and the bone-vascular links in chronic kidney disease. Eur J Clin Invest 36(s2):43–50
Portale A, Lonergan E, Tanney D, Halloran B (1997) Aging alters calcium regulation of serum concentration of parathyroid hormone in healthy men. Am J Physiol 272(1 Pt 1):E139-146
Ureña P, Kubrusly M, Mannstadt M, Hruby M, Trinh MM, Silve C, Lacour B, Abou-Samra AB, Segre GV, Drüeke T (1994) The renal PTH/PTHrP receptor is down-regulated in rats with chronic renal failure. Kidney Int 45(2):605 – 11
Galitzer H, Ben-Dov Z, Silver J, Naveh-Many T (2010) Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int 77(3):211–218
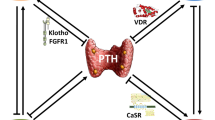
Komaba H, Goto S, Fujii H, Hamada Y, Kobayashi A, Shibuya K, Tominaga Y, Otsuki N, Nibu K, Nakagawa K, Tsugawa N, Okano T, Kitazawa R, Fukagawa M, Kita T (2010) Depressed expression of Klotho and FGF receptor 1 in hyperplastic parathyroid glands from uremic patients. Kidney Int 77(3):232–238
Brown A, Ritter C, Finch J, Slatopolsky E (1999) Decreased calcium-sensing receptor expression in hyperplastic parathyroid glands of uremic rats: role of dietary phosphate. Kidney Int 55(4):1284–1292
Brown A, Dusso A, Lopez-Hilker S, Lewis-Finch J, Grooms P, Slatopolsky E (1989) 1,25-(OH)2D receptors are decreased in parathyroid glands from chronically uremic dogs. Kidney Int 35(1):19–23
Mithal A, Kifor O, Kifor I, Vassilev P, Butters R, Krapcho K, Simin R, Fuller F, Hebert S, Brown E (1995) The reduced responsiveness of cultured bovine parathyroid cells to extracellular Ca2 + is associated with marked reduction in the expression of extracellular Ca(2+)-sensing receptor messenger ribonucleic acid and protein. Endocrinology 136(7):3087–3092
Ritter C, Finch J, Slatopolsky E, Brown A (2001, November) Parathyroid hyperplasia in uremic rats precedes down-regulation of the calcium receptor. Kidney Int 60(5):1737–1744
Fukuda N, Tanaka H, Tominaga Y, Fukagawa M, Kurokawa K, Seino Y (1993) Decreased 1,25-dihydroxyvitamin D3 receptor density is associated with a more severe form of parathyroid hyperplasia in chronic uremic patients. J Clin Invest 92(3):1436–1443
Silver J, Kilav R, Naveh-Many T (2002) Mechanisms of secondary hyperparathyroidism. Am J Physiol Renal Physiol 283(3):367–376
Román-García P, Carrillo-López N, Naves-Díaz M, Rodríguez I, Ortiz A, Cannata-Andía B (2012) Dual-specificity phosphatases are implicated in severe hyperplasia and lack of response to FGF23 of uremic parathyroid glands from rats. Endocrinology 153(4):1627–1637
DeFronzo RA, Alvestrand A, Smith D, Hendler R (1981) Insulin resistance in uremia. J Clin Invest 67:563—568
Blum WF, Ranke MB, Kietzmann K, Tonshoff B, Mehls (1991) Growth hormone resistance and inhibition of somatomedin activity by excess of insulin-like growth factor binding protein in uraemia. Pediatr Nephrol 5 0:539—544
Ketteler M, Block GA, Evenepoel P, Fukagawa M, Herzog CA, McCann L, Moe SM, Shroff R, Tonelli MA, Toussaint ND, Vervloet MG, Leonard MB (2017) Executive summary of the 2017 KDIGO chronic kidney disease-mineral and bone disorder (CKD-MBD) guideline update: what’s changed and why it matters. Kidney Int 92(1):26–36
Ketteler M, Elder G, Evenepoel P, Ix J, Jamal S, Lafage-Proust M, Shroff R, Thadhani R, Tonelli M, Kasiske B, Wheeler D, Leonard M (2015) Revisiting KDIGO clinical practice guideline on chronic kidney disease-mineral and bone disorder: a commentary from a kidney disease: improving global outcomes controversies conference. Kidney Int 87(3):502–528
Bover J, Ureña Torres P, Brandenburg V, Goldsmith D, Ruiz C, DaSilva I, Bosch RJ (2014) Adynamic bone disease: from bone to vessels in chronic kidney disease. Semin Nephrol 34(6):626–640
Haarhaus M, Monier-Faugere M, Magnusson P, Malluche H (2015) Bone alkaline phosphatase isoforms in hemodialysis patients with low versus non-low bone turnover: a diagnostic test study. Am J Kidney Dis 66(1):99–105
Magnusson P, Farley R (2002) Differences in sialic acid residues among bone alkaline phosphatase isoforms: a physical, biochemical, and immunological characterization. Calcif Tissue Int 71(6):508–518
Halling Linder C, Narisawa S, Millán J, Magnusson P (2009) Glycosylation differences contribute to distinct catalytic properties among bone alkaline phosphatase isoforms. Bone 45(5):987–993
Magnusson P, Sharp A, Magnusson M, Risteli J, Davie W, Larsson L (2001) Effect of chronic renal failure on bone turnover and bone alkaline phosphatase isoforms. Kidney Int 60(1):257–265
Swolin-Eide D, Hansson S, Larsson L, Magnusson P (2006) The novel bone alkaline phosphatase B1x isoform in children with kidney disease. Pediatr Nephrol 21(11):1723–1729
Haarhaus M, Fernström A, Magnusson M, Magnusson P (2009) Clinical significance of bone alkaline phosphatase isoforms, including the novel B1x isoform, in mild to moderate chronic kidney disease. Nephrol Dial Transplant 24(11):3382–3389
Haarhaus M, Arnqvist H, Magnusson P (2013) Calcifying human aortic smooth muscle cells express different bone alkaline phosphatase isoforms, including the novel B1x isoform. J Vasc Res 50(2):167–174
Jean G, Souberbielle J, Zaoui E, Lorriaux C, Mayor B, Hurot J, Deleaval P, Chazot C (2012) Total and bone-specific alkaline phosphatases in haemodialysis patients with chronic liver disease. Clin Biochem 45(6):436–439
Ureña-Torres P, De Vernejoul M (1999) Circulating biochemical markers of bone remodeling in uremic patients. Kidney Int 55(6):2141–2156
Damera S, Raphael L, Baird C, Cheung K, Greene T, Beddhu S (2011) Serum alkaline phosphatase levels associate with elevated serum C-reactive protein in chronic kidney disease. Kidney Int 79(2):228–233
Kunutsor K, Bakker J, Kootstra-Ros E, Gansevoort T, Gregson J, Dullaart P (2015) Serum alkaline phosphatase and risk of incident cardiovascular disease: interrelationship with high sensitivity C-reactive protein. PLoS ONE 10(7):1–16
Filipowicz R, Greene T, Wei G, Cheung A, Raphael K, Baird B, Beddhu S (2013) Associations of Serum Skeletal Alkaline Phosphatase with Elevated C-Reactive Protein and Mortality. Clin J Am Soc Nephrol 8:26–32
Kalantar-Zadeh K, Kuwae N, Regidor D, Kovesdy C, Kilpatrick R, Shinaberger C, McAllister C, Budoff M, Salusky I, Kopple J (2006) Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int 70:771–780
Blayney M, Pisoni P, Bragg-Gresham J, Bommer J, Piera L, Saito A, Akiba T, Keen M, Young E, Port F (2008) High alkaline phosphatase levels in hemodialysis patients are associated with higher risk of hospitalization and death. Kidney Int 74:655–663
Kovesdy C, Ureche V, Lu J, Kalantar-Zadeh K (2010) Outcome predictability of serum alkaline phosphatase in men with pre-dialysis CKD. Nephrol Dial Transplant 25:3003–3011
Taliercio J, Schold J, Simon J, Arrigain S, Tang A, Saab S, Nally J, Navaneethan S (2013) Prognostic importance of serum alkaline phosphatase in CKD stages 3–4 in a clinical population. Am J Kidney Dis 62(4):703–710
Molnar M, Kovesdy C, Mucsi I, Salusky I, Kalantar-Zadeh K (2012) Association of pre–kidney transplant markers of mineral and bone disorder with post-transplant outcomes. Clin J Am Soc Nephrol 7:1859–1871
Beddhu S, Ma X, Baird B, Cheung A, Greene T (2009) Serum alkaline phosphatase and mortality in African Americans with chronic kidney disease. Clin J Am Soc Nephrol 4:1805–1810
Rhee C, Molnar M, Lau W, Ravel V, Kovesdy C, Mehrotra R, Kalantar–Zadeh K (2014) Comparative mortality-predictability using alkaline phosphatase and parathyroid hormone in patients on peritoneal dialysis and hemodialysis. Perit Dial Int 34:732–748
Lertdumrongluk P, Lau W, Park J, Rhee C, Kovesdy C, Kalantar-Zadeh K (2013) Impact of age on survival predictability of bone turnover markers in hemodialysis patients. Nephrol Dial Transplant 28:2535–2545
Kobayashi I, Shidara K, Okuno S, Yamada S, Imanishi Y, Mori K, Ishimura E, Shoji S, Yamakawa T, Inaba M (2012) Higher serum bone alkaline phosphatase as a predictor of mortality in male hemodialysis patients. Life Sci 90(5–6):212–218
Chang J, Feng Y, Peng Y, Hsu S, Pai M, Chen H, Wu H, Yang J (2014) Combined alkaline phosphatase and phosphorus levels as a predictor of mortality in maintenance hemodialysis patients. Medicine (Baltimore) 93(18):1–8
Sumida K, Molnar M, Potukuchi P, Thomas F, Lu J, Obi Y, Rhee C, Streja E, Yamagata K, Kalantar-Zadeh K, Kovesdy C (2017) Prognostic significance of pre-end-stage renal disease serum alkaline phosphatase for post-end-stage renal disease mortality in late-stage chronic kidney disease patients transitioning to dialysis. Nephrol Dial Transplant. https://doi.org/10.1093/ndt/gfw412 [E-pub ahead of print]
Tonelli M, Curhan G, Pfeffer M, Sacks F, Thadhani R, Melamed M, Wiebe N, Muntner P (2009) Relation between alkaline phosphatase, serum phosphate, and all-cause or cardiovascular mortality. Circulation 120(18):1784–1792
Drechsler C, Verduijn M, Pilz S, Krediet R, Dekker F, Wanner C, Ketteler M, Boeschoten E, Brandenburg V, NECOSAD Study Group (2011). Bone alkaline phosphatase and mortality in dialysis patients. Clin J Am Sc Nephrol 6 (7): 1752–1759
Maruyama Y, Taniguchi M, Kazama J, Yokoyama K, Hosoya T, Yokoo T, Shigematsu T, Iseki K, Tsubakihara Y (2014) A higher serum alkaline phosphatase is associated with the incidence of hip fracture and mortality among patients receiving hemodialysis in Japan. Nephrol Dial Transplant 29(8):1532–1538
Shantouf R, Kovesdy C, Kim Y, Ahmadi N, Luna A, Luna C, Rambod M, Nissenson A, Budoff M, Kalantar-Zadeh K (2009) Association of serum alkaline phosphatase with coronary artery calcification in maintenance hemodialysis patients. Clin J Am Soc Nephrol 4(6):1106–1114
Chen J, Mohler E, Xie D, Shlipak M, Townsend R, Appel L, Ojo A, Schreiber M, Nessel L, Zhang X, Raj D, Strauss L, Lora C, Rahman M, Hamm L, He J, CRIC Study Investigators. (2016). Traditional and non-traditional risk factors for incident peripheral arterial disease among patients with chronic kidney disease. Nephrol Dial Transplant 31 (7): 1145–1151
Park J, Kovesdy C, Duong U, Streja E, Rambod M, Nissenson A, Sprague S, Kalantar-Zadeh K (2010) Association of serum alkaline phosphatase and bone mineral density in maintenance hemodialysis patients. Hemodial Int 14(2):182–192
Kalantar-Zadeh K, Lee G, Miller J, Streja E, Jing J, Robertson J, Kovesdy C (2009) Predictors of hyporesponsiveness to erythropoiesis-stimulating agents in hemodialysis patients. Am J Kidney Dis 53(5):823–834
Bergman A, Qureshi A, Haarhaus M, Lindholm B, Barany P, Heimburger O, Stenvinkel P, Anderstam B (2016) Total and bone-specific alkaline phosphatase are associated with bone mineral density over time in end-stage renal disease patients starting dialysis. J Nephrol 1:1–8
Regidor D, Kovesdy C, Mehrotra R, Rambod M, Jing J, McAllister C, Van Wyck D, Kopple J, Kalantar-Zadeh K (2008) Serum alkaline phosphatase predicts mortality among maintenance hemodialysis patients. J Am Soc Nephrol 19(11):2193 – 203
Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, Kronenberg F, Marcelli D, Passlick-Deetjen J, Schernthaner G, Fouqueray B, Wheeler D, ARO Investigators (2011). Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant 26(6): 1948–1955
Naves M, Passlick J, Guinsburg A, Marelli C, Fernández J, Rodríguez D, Cannata J (2011) Calcium, phosphorus, PTH and death rates in a large sample of dialysis patients from Latin America. The CORES Study. Nephrol Dial Transplant 26(6):1938–1947
Fernández-Martín JL, Martínez-Camblor P, Dionisi MP, Floege J, Ketteler M, London G, Locatelli F, Gorriz JL, Rutkowski B, Ferreira A, Bos WJ, Covic A, Rodríguez-García M, Sánchez JE, Rodríguez-Puyol D, Cannata-Andia JB; COSMOS group (2015). Improvement of mineral and bone metabolism markers is associated with better survival in haemodialysis patients: the COSMOS study. Nephrol Dial Transplant 30(9):1542–1551
Lau W, Kalantar-Zadeh K, Kovesdy C, Mehrotra R (2014) Alkaline phosphatase: better than PTH as a marker of cardiovascular and bone disease? Hemodial Int 18(4):720–724
Fahrleitner-Pammer A, Herberth J, Browning S, Obermayer-Pietsch B, Wirnsberger G, Holzer H, Dobnig H, Malluche H (2008) Bone markers predict cardiovascular events in chronic kidney disease. J Bone Miner Res 23(11):1850–1858
Robinson-Cohen C, Katz R, Hoofnagle A, Cauley J, Furberg C, Robbins J, Chen Z, Siscovick D, de Boer I, Kestenbaum B (2011) Mineral metabolism markers and the long-term risk of hip fracture: the cardiovascular health study. J Clin Endocrinol Metab 96(7):2186–2193
David C, Bover J, Voiculet C, Peride I, Petcu L, Niculae A, Covic A, Checherita I (2016) Coronary risk score for mineral bone disease in chronic non-diabetic hemodialysis patients: results from a prospective pilot study. Int Urol Nephrol 18:1–12
Beige J, Wendt R, Girndt M, Queck K, Fiedler R, Jehle P (2014) Association of serum alkaline phosphatase with mortality in non-selected European patients with CKD5D: an observational, three-centre survival analysis. BMJ Open 4(2):1–7
Soohoo M, Feng M, Obi Y, Streja E, Rhee C, Lau W, Wang J, Ravel V, Brunelli S, Kovesdy C, Kalantar-Zadeh K (2016) Changes in Markers of Mineral and Bone Disorders and Mortality in Incident Hemodialysis Patients. Am J Nephrol 43(2):85–96
Qiao J, Mertens R, Fishbein M, Geller SA (2003) Cartilaginous metaplasia in calcified diabetic peripheral vascular disease: Morphologic evidence of enchondral ossification. Human Pathol 34:402–407
Shroff R, McNair R, Figg N, Skepper J, Schurgers L, Gupta A, Hiorns M, Donald A, Deanfield J, Rees L, Shanahan C (2008) Dialysis Accelerates Medial Vascular Calcification in Part by Triggering Smooth Muscle Cell Apoptosis. Circulation 118:748–1757
El-Abbadi M, Pai A, Leaf E, Yang H, Bartley B, Quan K, Ingalls C, Liao H, Giachelli C (2009) Phosphate feeding induces arterial medial calcification in uremic mice: role of serum phosphorus, fibroblast growth factor-23, and osteopontin. Kidney Int 75:1297–1307
Nakano-Kurimoto R, Ikeda K, Uraoka M, Nakagawa Y, Yutaka K, Koide M, Takahashi T, Matoba S, Yamada H, Okigaki M, Matsubara H (2009) Replicative senescence of vascular smooth muscle cells enhances the calcification through initiating the osteoblastic transition. Am J Physiology Heart Circ Physiology 297(5):1673–1684
Zhu D, Mackenzie N, Millán J, Farquharson C, MacRae V (2011) The appearance and modulation of osteocyte marker expression during calcification of vascular smooth muscle cells. PLoS ONE 6(5):1–10
Shioi A, Katagi M, Okuno Y, Mori K, Jono S, Koyama H, Nishizawa Y (2002) Induction of bone-type alkaline phosphatase in human vascular smooth muscle cells: roles of tumor necrosis factor-alpha and oncostatin M derived from macrophages. Circulation Res 91(1):9–16
Sheen C, Kuss P, Narisawa S, Yadav M, Nigro J, Wang W, Chhea T, Sergienko E, Kapoor K, Jackson M, Hoylaerts M, Pinkerton A, O’Neill W, Millán J (2015) Pathophysiological role of vascular smooth muscle alkaline phosphatase in medial artery calcification. J Bone Miner Res 30(5):824–836
Savinov Y, Salehi M, Yadav C, Radichev I, Millán L, Savinova V (2015) Transgenic overexpression of tissue-nonspecific alkaline phosphatase (TNAP) in vascular endothelium results in generalized arterial calcification. J Am Heart Assoc 4(12):1–13
Schoppet M, Shanahan M (2008) Role for alkaline phosphatase as an inducer of vascular calcification in renal failure? Kidney Int 73(9):989–991
Murshed M, Harmey D, Millán L, McKee D, Karsenty G (2005) Unique coexpression in osteoblast of broadly expressed genes accounts for the spatial restriction of ECM mineralization to bone. Genes Dev 19(9):1093–1104
Watson E, Parhami F, Shin V, Demer L (1998) Fibronectin and collagen I matrixes promote calcification of vascular cells in vitro, whereas collagen IV matrix is inhibitory. Arterioescler Thromb Vasc Biol 18(12):1964–1971
Ishimura E, Okuno S, Okazaki H, Norimine K, Yamakawa K, Yamakawa T, Shoji S, Nishizawa Y, Inaba M (2014) Significant association between bone-specific alkaline phosphatase and vascular calcification of the hand arteries in male hemodialysis patients. Kidney Blood Press Res 39:299–307
Iba K, Takada J, Yamashita T (2004) The serum level of bone-specific alkaline phosphatase activity is associated with aortic calcification in osteoporosis patients. J Bone Miner Metab 22(6):594–596
Lomashvili K, Narisawa S, Millán J, O’Neill W (2014) Vascular calcification is dependent on plasma levels of pyrophosphate. Kidney Int 85(6):1351–1356
Joubert P, Ketteler M, Salcedo C, Perelló J (2016) Hypothesis: phytate is an important unrecognised nutrient and potential intravenous drug for preventing vascular calcification. Med Hypotheses 94:89–92
Buades J, Sanchís P, Perelló J, Grases F (2016) Plant phosphates, phytate and pathological calcifications in chronic kidney disease. Nefrología 30:1–9
Bover J, Ureña Torres P, Górriz J, Lloret M, da Silva I, Ruiz-García C, Chang P, Rodríguez M (2016) Cardiovascular calcifications in chronic kidney disease: potential therapeutic implications. Ballarín J Nefrología, 36(6):597–608
Narisawa S, Harmey D, Yadav M, O’Neill W, Hoylaerts M, Millán J (2007) Novel inhibitors of alkaline phosphatase suppress vascular smooth muscle cell calcification. J Bone Miner Res 11:1700–1710
Dahl R, Sergienko E, Su Y, Mostofi Y, Yang L, Simao A, Narisawa S, Brown B, Mangravita-Novo A, Vicchiarelli M, Smith L, O’Neill W, Millán J, Cosford N (2009) Discovery and validation of a series of aryl sulfonamides as selective inhibitors of tissue-nonspecific alkaline phosphatase (TNAP). J Medicinal Chemistry 21:6919–6925
Sidique S, Ardecky R, Su Y, Narisawa S, Brown B, Millán J, Sergienko E, Cosford N (2009) Design and synthesis of pyrazole derivatives as potent and selective inhibitors of tissue-nonspecific alkaline phosphatase (TNAP). Bioorg Med Chem Lett 19(1):222–225
Sergienko E, Su Y, Chan X, Brown B, Hurder A, Narisawa S, Millán J (2009) Identification and characterization of novel tissue-nonspecific alkaline phosphatase inhibitors with diverse modes of action. J Biomol Screen 7:824–837
Chung T, Sergienko E, Millán J (2010) Assay format as a critical success factor for identification of novel inhibitor chemotypes of tissue-nonspecific alkaline phosphatase from high-throughput screening. Molecules 5:3010–3037
Sergienko E, Millán J (2010) High-throughput screening of tissue-nonspecific alkaline phosphatase for identification of effectors with diverse modes of action. Nat Protoc 8:1431–1439
Linder C, Ek-Rylander B, Krumpel M, Norgård M, Narisawa S, Millán J, Andersson G, Magnusson P (2017) Bone alkaline phosphatase and tartrate-resistant acid phosphatase: potential co-regulators of bone mineralization. Calcif Tissue Int 101(1):92–101
Boström K, Tsao D, Shen S, Wang Y, Demer L (2001) Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem 276(17):14044–14052
Rawadi G, Vayssière B, Dunn F, Baron R, Roman-Roman S (2003) BMP-2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res 18(10):1842–1853
Murali S, Roschger P, Zeitz U, Klaushofer K, Andrukhova O, Erben R (2016) FGF23 regulates bone mineralization in a 1,25(OH)2 D3 and Klotho-Independent manner. J Bone Miner Res 31(1):129–142
Murali S, Andrukhova O, Clinkenbeard E, White K, Erben R (2016) Excessive Osteocytic FGF23 Secretion Contributes to Pyrophosphate Accumulation and Mineralization Defect in Hyp Mice. PLoS Biol 14(4):1–24
Martin S, Lin H, Ejimadu C, Lee T (2015) Tissue-nonspecific alkaline phosphatase as a target of sFRP2 in cardiac fibroblasts. Am J Physiol Cell Physiol 3:139–147
Ali A, Penny C, Paiker J, Psaras G, Ikram F, Crowther N (2006) The effect of alkaline phosphatase inhibitors on intracellular lipid accumulation in preadipocytes isolated from human mammary tissue. Ann Clin Biochem 43:207–213
Sardiwal S, Magnusson P, Goldsmith D, Lamb E (2013) Bone alkaline phosphatase in CKD-mineral bone disorder. Am J Kidney Dis 4:810–822
Cheung C, Tan K, Lam K, Cheung B (2013) The relationship between glucose metabolism, metabolic syndrome, and bone-specific alkaline phosphatase: a structural equation modeling approach. J Clin Endocrinol Metab 98:3856–3863
Kaliannan K, Hamarneh R, Economopoulos P, Nasrin S, Moaven O, Patel P, Malo S, Ray M, Abtahi M, Muhammad N, Raychowdhury A, Teshager A, Mohamed M, Moss K, Ahmed R, Hakimian S, Narisawa S, Millán L, Hohmann E, Warren S, Bhan K, Malo S, Hodin A (2013) Intestinal alkaline phosphatase prevents metabolic syndrome in mice. Proc Nat Acad Sci USA 110(17):7003–7008
Malo S (2015) A high level of intestinal alkaline phosphatase is protective against Type 2 diabetes mellitus irrespective of obesity. EBioMedicine 2(12):2016–2023
Lips P, Duong T, Oleksik A, Black D, Cummings S, Cox D, Nickelsen T (2001) A global study of vitamin D status and parathyroid function in postmenopausal women with osteoporosis: baseline data from the multiple outcomes of raloxifene evaluation clinical trial. J Clin Endocrinol Metab 86:3008
Thomas M, Lloyd-Jones D, Thadhani R, Shaw A, Deraska D, Kitch B, Vamvakas E, Dick I, Prince R, Finkelstein J (1998) Hypovitaminosis D in medical inpatients. N Eng J Med 338:777–783
Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, Steele D, Chang Y, Camargo C, Tonelli M, Thadhani R (2007) Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int 72(8):1004–1013
Belozeroff V, Goodman W, Ren L, Kalantar-Zadeh K (2009) Cinacalcet lowers serum alkaline phosphatase in maintenance hemodialysis patients. Clin J Am Soc Nephrol 4(3):673–679
Llach F, Yudd M (2001) Paricalcitol in dialysis patients with calcitriol-resistant secondary hyperparathyroidism. Am J Kidney Dis 38(5 Suppl 5):45–50
Palmer S, McGregor D, Macaskill P, Craig J, Elder G, Strippoli F (2007) Meta-analysis: vitamin D compounds in chronic kidney disease. Ann Intern Med 147(12):840–853
Coyne D, Andress D, Amdahl M, Ritz E, de Zeeuw D (2013) Effects of paricalcitol on calcium and phosphate metabolism and markers of bone health in patients with diabetic nephropathy: results of the VITAL study. Nephrol Dial Transplant 28(9):2260–2268
Lomashvili K, Khawandi W, O’Neill C (2005) Reduced plasma pyrophosphate levels in hemodialysis patients. J Am Soc Nephrol 16(8):2495–2500
Makar H, Sawires K, Farid M, Ali M, Schaalan M (2010) Effect of high-flux versus low-flux dialysis membranes on parathyroid hormone. Iran J Kidney Dis 4(4):327–332
López-González A, Grases F, Perello J, Tur F, Costa A, Monroy N, Mari B, Vicente T (2010) Phytate levels and bone parameters: a retrospective pilot clinical trial. Front Biosci 2:1093–1098
Grases F, Sanchis P, Perello J, Isern B, Prieto R, Fernandez-Palomeque C, Fiol M, Bonnin O, Torres J (2006). Phytate (Myo-inositol hexakisphosphate) inhibits cardiovascular calcifications in rats. Front Biosci, (11): 136–142
Perelló J, Salcedo C, Joubert P, Canals A, Ferrer M (2015) First-time-in-human phase 1 clinical trial in healthy volunteers with SNF472, a novel inhibitor of vascular calcification. Nephrol Dial Transplant 30(suppl 3):iii592 (abstract)
Jansen R, Duijst S, Mahakena S, Sommer D, Szeri F, Váradi A, Plomp A, Bergen A, Oude R, Borst P, van de Wetering K (2014) ABCC6-mediated ATP secretion by the liver is the main source of the mineralization inhibitor inorganic pyrophosphate in the systemic circulation-brief report. Arterioscler Thromb Vasc Biol 34(9):1985–1989
Pomozi V, Brampton C, Szeri F, Dedinszki D, Kozák E, van de Wetering K, Hopkins H, Martin L, Váradi A Le Saux (2016) Functional rescue of ABCC6 deficiency by 4-Phenylbutyrate therapy reduces dystrophic calcification in Abcc6-/- Mice. J Invest Dermatol 5:1–25
Albright R, Stabach P, Cao W, Kavanagh D, Mullen I, Braddock A, Covo M, Tehan M, Yang G, Cheng Z, Bouchard K, Yu Z, Thorn S, Wang X, Folta-Stogniew E, Negrete A, Sinusas A, Shiloach J, Zubal G, Madri J, De La Cruz E, Braddock D (2015) ENPP1-Fc prevents mortality and vascular calcifications in rodent model of generalized arterial calcification of infancy. Albright Nature Commun 6:1–32
Ho A, Johnson M, Kingsley D (2000) Role of the mouse ank gene in control of tissue calcification and arthritis. Science 289(5477):265–270
Wang W, Xu J, Du B, Kirsch T (2005) Role of the progressive ankylosis gene (ank) in cartilage mineralization. Molecular Cellular Biology 25(1):312–323
Gurley K, Chen H, Guenther C, Nguyen E, Rountree R, Schoor M, Kingsley D (2006) Mineral formation in joints caused by complete or joint-specific loss of ANK function. J Bone Miner Res 21(8):1238–1247
Villa-Bellosta R, Rivera-Torres J, Osorio F, Acín-Pérez R, Enriquez J, López-Otín C, Andrés V (2013) Defective extracellular pyrophosphate metabolism promotes vascular calcification in a mouse model of Hutchinson-Gilford progeria syndrome that is ameliorated on pyrophosphate treatment. Circulation 127(24):2442–2451
De Oliveira R, Louvet L, Riser B, Barreto F, Benchitrit J, Rezg R, Poirot S, Jorgetti V, Drüeke T, Massy Z (2015) Peritoneal delivery of sodium pyrophosphate blocks the progression of pre-existing vascular calcification in uremic apolipoprotein-E knockout mice. Calcif Tissue Int 97(2):179 – 92
Marqués S, Buchet R, Popowycz F, Lemaire M, Mebarek S (2016) Synthesis of benzofuran derivatives as selective inhibitors of tissue-nonspecific alkaline phosphatase: effects on cell toxicity and osteoblast-induced mineralization. Bioorg Med Chem Lett 26(5):1457–1459
Picaud S, Wells C, Felletar I, Brotherton D, Martin S, Savitsky P, Diez-Dacal B, Philpott M, Bountra C, Lingard H, Fedorov O, Müller S, Brennan E, Knapp S, Filippakopoulos P (2013) RVX-208, an inhibitor of BET transcriptional regulators with selectivity for the second bromodomain. Proc Nat Acad Sci USA 110(49):19754–19759
Gilham D, Wasiak S, Tsujikawa L, Halliday C, Norek K, Patel R, Kulikowski E, Johansson J, Sweeney M, Wong N (2016) RVX-208, a BET-inhibitor for treating atherosclerotic cardiovascular disease, raises ApoA-I/HDL and represses pathways that contribute to cardiovascular disease. Atheroscler 247:48–57
Kalantar-Zadeh K et al (2015) Alkaline phosphatase lowering by selective bet inhibition, a novel mechanism for mace reduction in high risk cvd, diabetes and CKD patients — a post-hoc analysis of phase 2b studies with RVX-208. J Am Soc Nephrol 26:227A
Wong N, Kalantar-Zadeh K, Kulikowski E, Wasiak S, Gilham D, Halliday C, Sweeney M, Johansson J (2016) SP071 Apabetalone (RVX-208), a selective bromodomain and extra-terminal (BET) protein inhibitor, decreases abundance and activity of complement proteins in vitro, in mice and in clinical studies. Nephrol Dial Transplant 31(suppl 1):i109
Kausik R (Estimated study completion data October 2018). Phase A, Multi-Center III, Double-Blind, Randomized, Parallel Group, Placebo-Controlled Clinical Trial in High-Risk Type 2 Diabetes Mellitus (T2DM) Subjects With Coronary Artery Disease (CAD) to Determine Whether Bromodomain Extraterminal Domain (BET) Inhibition Treatment With RVX000222 Increases. the Time to Major Adverse Cardiovascular Events (MACE)
Gasque C, Foster L, Kuss P, Yadav C, Liu J, Kiffer-Moreira T, van Elsas A, Hatch N, Somerman J, Millán L (2015) Improvement of the skeletal and dental hypophosphatasia phenotype in Alpl-/- mice by administration of soluble (non-targeted) chimeric alkaline phosphatase. Bone 72:137–147
Peters E, Mehta L, Murray T, Hummel J, Joannidis M, Kellum A, Arend J, Pickkers P (2016) Study protocol for a multicentre randomised controlled trial: Safety, Tolerability, efficacy and quality of life Of a human recombinant alkaline Phosphatase in patients with sepsis-associated Acute Kidney Injury (STOP-AKI). BMJ Open 6(9):e012371
Peters E, Geraci S, Heemskerk S, Wilmer J, Bilos A, Kraenzlin B, Gretz N, Pickkers P, Masereeuw R (2015) Alkaline phosphatase protects against renal inflammation through dephosphorylation of lipopolysaccharide and adenosine triphosphate. Br J Pharmacol 172(20):4932–4945
Peters E, Ergin B, Kandil A, Gurel-Gurevin E, van Elsas A, Masereeuw R, Pickkers P, Ince C (2016) Effects of a human recombinant alkaline phosphatase on renal hemodynamics, oxygenation and inflammation in two models of acute kidney injury. Toxicol Appl Pharmacol 313:88–96
Ghosh S, Gehr W, Ghosh S (2014) Curcumin and chronic kidney disease (CKD): major mode of action through stimulating endogenous intestinal alkaline phosphatase. Molecules 19(12):20139–20156
Ghosh S, Bie J, Wang J, Ghosh S (2014) Oral supplementation with non-absorbable antibiotics or curcumin attenuates western diet-induced atherosclerosis and glucose intolerance in LDLR-/- mice–role of intestinal permeability and macrophage activation. PLoS One 9(9):e108577: 1–9
Millán L, Whyte P (2016) Alkaline Phosphatase and Hypophosphatasia. Calcif Tissue Int 98(4):398–416
Sardiwal S, Gardham C, Coleman A, Stevens P, Delaney M, Lamb E (2012) Bone-specific alkaline phosphatase concentrations are less variable than those of parathyroid hormone in stable hemodialysis patients. Kidney Int 82(1):100–105
Garrett G, Sardiwal S, Lamb E, Goldsmith D (2013) PTH–a particularly tricky hormone: why measure it at all in kidney patients? Clin J Am Soc Nephrol 8(2):299–312
Sprague S, Moe S (2013) The Case for Routine Parathyroid Hormone Monitoring. Clin J Am Soc Nephrol 8(2):313–318
Gardham C, Stevens E, Delaney P, LeRoux M, Coleman A, Lamb J (2010) Variability of parathyroid hormone and other markers of bone mineral metabolism in patients receiving hemodialysis. Clin J Am Soc Nephrol 5(7):1261–1267
Acknowledgements
Dr Jordi Bover belongs to the Spanish National Network of Kidney Research RedinRen (RD06/0016/0001 and RD12/0021/0033) and the Spanish National Biobank network RD09/0076/00064. Dr Jordi Bover also belongs to the Catalan Nephrology Research Group AGAUR 2009 SGR-1116 and collaborates with the Spanish Fundación Iñigo Alvarez de Toledo (FRIAT). We thank Mr. Ricardo Pellejero for his invaluable bibliographic assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Bover, J., Ureña, P., Aguilar, A. et al. Alkaline Phosphatases in the Complex Chronic Kidney Disease-Mineral and Bone Disorders. Calcif Tissue Int 103, 111–124 (2018). https://doi.org/10.1007/s00223-018-0399-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-018-0399-z