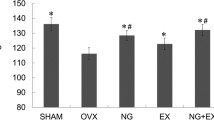
Abstract
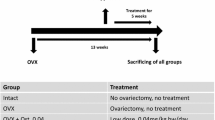
Osteoporosis is often accompanied by sarcopenia. The effect of strontium ranelate (SR) on muscle tissue has not been investigated sufficiently. In this study, the effect of different SR treatments on muscle was studied. Additionally, the lumbar vertebrae were analyzed. Three-month-old female rats were divided into five groups (n = 12): Group 1: untreated (NON-OVX); Group 2: ovariectomized and left untreated (OVX); Group 3: SR after OVX until the study ended (13 weeks, SR prophylaxis and therapy = pr+th); Group 4: OVX and SR for 8 weeks (SR prophylaxis = pr); Group 5: SR for 5 weeks from the 8 week after OVX (SR therapy = SR th). SR was applied in food (630 mg/kg body weight). The size of muscle fibers, capillary density, metabolic enzymes, and mRNA expression were assessed in soleus, gastrocnemius, and longissimus muscles. The vertebral bodies underwent micro-CT, biomechanical, and ashing analyses. In general, SR did not alter the muscle histological parameters. The changes in fiber size and capillary ratio were related to the body weight. Myostatin mRNA was decreased in Sr pr+th; protein expression was not changed. SR th led to increase in mRNA expression of vascular endothelial growth factor (Vegf-B). In lumbar spine, SR pr+th enhanced biomechanical properties, bone mineral density, trabecular area, density, and thickness and cortical density. The reduced calcium/phosphate ratio in the SR pr+th group indicates the replacement of calcium by strontium ions. SR has no adverse effects on muscle tissue and it shows a favorable time-dependent effect on vertebrae. A functional analysis of muscles could verify these findings.









Similar content being viewed by others
References
Satpathy S, Patra A, Ahirwar B (2015) Experimental techniques for screening of antiosteoporotic activity in postmenopausal osteoporosis. J Complement Integr Med 12(4):251–266. https://doi.org/10.1515/jcim-2015-0034
Johnell O, Kanis JA (2006) An estimate of the worldwide prevalence and disability associated with osteoporotic fractures. Osteoporos Int 17(12):1726–1733. https://doi.org/10.1007/s00198-006-0172-4
ROLLAND Y, CZERWINSKI S, van KAN, GA et al (2008) Sarcopenia: Its assessment, etiology, pathogenesis, consequences and future perspectives. J Nutr Health Aging 12(7):433–450
Bruyère O, Beaudart C, Locquet M et al (2016) Sarcopenia as a public health problem. Eur Geriatric Med 7(3):272–275. https://doi.org/10.1016/j.eurger.2015.12.002
Kanis JA, McCloskey EV, Johansson H et al (2013) European guidance for the diagnosis and management of osteoporosis in postmenopausal women. Osteoporos Int 24(1):23–57. https://doi.org/10.1007/s00198-012-2074-y
DVO (2014) OSTEOPOROSE bei Männern ab dem 60. Lebensjahr und bei postmenopausalen Frauen: Leitlinie des Dachverbands der Deutschsprachigen Wissenschaftlichen Osteologischen Gesellschaften e.V
Reginster JY, Seeman E, Vernejoul MC de et al (2005) Strontium ranelate reduces the risk of nonvertebral fractures in postmenopausal women with osteoporosis: treatment of Peripheral Osteoporosis (TROPOS) study. J Clin Endocrinol Metab 90(5):2816–2822. https://doi.org/10.1210/jc.2004-1774
Kanis JA, Johansson H, Oden A et al (2011) A meta-analysis of the effect of strontium ranelate on the risk of vertebral and non-vertebral fracture in postmenopausal osteoporosis and the interaction with FRAX((R)). Osteoporos Int 22(8):2347–2355. https://doi.org/10.1007/s00198-010-1474-0
Meunier PJ, Roux C, Seeman E et al (2004) The effects of strontium ranelate on the risk of vertebral fracture in women with postmenopausal osteoporosis. N Engl J Med 350(5):459–468. https://doi.org/10.1056/NEJMoa022436
Reginster JY, Brandi ML, Cannata-Andia J et al (2015) The position of strontium ranelate in today’s management of osteoporosis. Osteoporos Int 26(6):1667–1671. https://doi.org/10.1007/s00198-015-3109-y
Reid IR (2015) Efficacy, effectiveness and side effects of medications used to prevent fractures. J Intern Med 277(6):690–706. https://doi.org/10.1111/joim.12339
Reginster JY (2014) Cardiac concerns associated with strontium ranelate. Expert Opin Drug Saf 13(9):1209–1213. https://doi.org/10.1517/14740338.2014.939169
Maeda SS, Lazaretti-Castro M (2014) An overview on the treatment of postmenopausal osteoporosis. Arq Bras Endocrinol Metabol 58(2):162–171
Bakker AD, Zandieh-Doulabi B, Klein-Nulend J (2013) Strontium ranelate affects signaling from mechanically-stimulated osteocytes towards osteoclasts and osteoblasts. Bone 53(1):112–119. https://doi.org/10.1016/j.bone.2012.11.044
Li YF, Luo E, Feng G et al (2010) Systemic treatment with strontium ranelate promotes tibial fracture healing in ovariectomized rats. Osteoporos Int 21(11):1889–1897. https://doi.org/10.1007/s00198-009-1140-6
Ozturan KE, Demir B, Yucel I et al (2011) Effect of strontium ranelate on fracture healing in the osteoporotic rats. J Orthop Res 29(1):138–142. https://doi.org/10.1002/jor.21204
Ammann P, Shen V, Robin B et al (2004) Strontium ranelate improves bone resistance by increasing bone mass and improving architecture in intact female rats. J Bone Miner Res 19(12):2012–2020. https://doi.org/10.1359/JBMR.040906
Komrakova M et al (2015) The impact of strontium ranelate on metaphyseal bone healing in ovariectomized rats. Calcif Tissue Int 97(4):391–401. https://doi.org/10.1007/s00223-015-0019-0
Komrakova M, Hoffmann DB, Nuehnen V et al (2016) The effect of vibration treatments combined with teriparatide or strontium ranelate on bone healing and muscle in ovariectomized rats. Calcif Tissue Int 99(4):408–422. https://doi.org/10.1007/s00223-016-0156-0
Molinuevo MS, Fernandez JM, Cortizo AM et al (2017) Advanced glycation end products and strontium ranelate promote osteogenic differentiation of vascular smooth muscle cells in vitro: preventive role of vitamin D. Mol Cell Endocrinol 450:94–104. https://doi.org/10.1016/j.mce.2017.04.022
Servier Laboratories Limited (2016) Protelos: summary of product characteristics (SPC)–(eMC). https://www.medicines.org.uk/emc/medicine/15410. Accessed 24 Nov 2016
Cunha-Henriques S, Costa-Paiva L, Pinto-Neto AM et al (2011) Postmenopausal women with osteoporosis and musculoskeletal status: a comparative cross-sectional study. J Clin Med Res 3(4):168–176. https://doi.org/10.4021/jocmr537w
Kaji H (2013) Linkage between muscle and bone: common catabolic signals resulting in osteoporosis and sarcopenia. Curr Opin Clin Nutr Metab Care 16(3):272–277. https://doi.org/10.1097/MCO.0b013e32835fe6a5
Sjoblom S, Suuronen J, Rikkonen T et al (2013) Relationship between postmenopausal osteoporosis and the components of clinical sarcopenia. Maturitas 75(2):175–180. https://doi.org/10.1016/j.maturitas.2013.03.016
He H, Liu Y, Tian Q et al (2015) Relationship of sarcopenia and body composition with osteoporosis. Osteoporos Int. https://doi.org/10.1007/s00198-015-3241-8
Iwasa T, Matsuzaki T, Tungalagsuvd A et al (2014) Effects of ovariectomy on the inflammatory responses of female rats to the central injection of lipopolysaccharide. J Neuroimmunol 277(1–2):50–56. https://doi.org/10.1016/j.jneuroim.2014.09.017
Habermann B, Kafchitsas K, Olender G et al (2010) Strontium ranelate enhances callus strength more than PTH 1–34 in an osteoporotic rat model of fracture healing. Calcif Tissue Int 86(1):82–89. https://doi.org/10.1007/s00223-009-9317-8
Saul D, Kling JH, Kosinsky RL et al (2016) Effect of the lipoxygenase inhibitor baicalein on muscles in ovariectomized rats. J Nutr Metab. https://doi.org/10.1155/2016/3703216
Komrakova M, Krischek C, Wicke M et al (2011) Influence of intermittent administration of parathyroid hormone on muscle tissue and bone healing in orchiectomized rats or controls. J Endocrinol 209(1):9–19. https://doi.org/10.1530/JOE-10-0353
Andersen P (1975) Capillary density in skeletal muscle of man. Acta Physiol Scand 95(2):203–205. https://doi.org/10.1111/j.1748-1716.1975.tb10043.x
Komrakova M, Werner C, Wicke M et al (2009) Effect of daidzein, 4-methylbenzylidene camphor or estrogen on gastrocnemius muscle of osteoporotic rats undergoing tibia healing period. J Endocrinol 201(2):253–262. https://doi.org/10.1677/JOE-08-0521
Horák V (1983) A successive histochemical staining for succinate dehydrogenase and ?: Reversed?-ATPase in a single section for the skeletal muscle fibre typing. Histochemistry 78(4):545–553. https://doi.org/10.1007/BF00496207
Faloona GR, Srere PA (1969) Escherichia coli citrate synthase: purification and the effect of potassium on some properties. Biochemistry 8(11):4497–4503. https://doi.org/10.1021/bi00839a041
Hatefi Y, Stiggall DL (1978) [2] Preparation and properties of NADH: Cytochrome c oxidoreductase (complex I–III). In: Fleischer S (ed) Biological oxidations: mitochondrial and microbial systems, vol 53. Academy Press, New York, pp 5–10
Spandidos A, Wang X, Wang H et al (2010) PrimerBank: a resource of human and mouse PCR primer pairs for gene expression detection and quantification. Nucleic Acids Res 38(Database issue):D792–D799. https://doi.org/10.1093/nar/gkp1005
Spandidos A, Wang X, Wang H et al (2008) A comprehensive collection of experimentally validated primers for polymerase chain reaction quantitation of murine transcript abundance. BMC Genome 9:633. https://doi.org/10.1186/1471-2164-9-633
Wang X, Seed B (2003) A PCR primer bank for quantitative gene expression analysis. Nucleic Acids Res 31(24):e154
Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25(4):402–408. https://doi.org/10.1006/meth.2001.1262
Sehmisch S, Erren M, Rack T et al (2009) Short-term effects of parathyroid hormone on rat lumbar vertebrae. Spine 34(19):2014–2021. https://doi.org/10.1097/BRS.0b013e3181afe846
Sehmisch S, Erren M, Kolios L et al (2010) Effects of isoflavones equol and genistein on bone quality in a rat osteopenia model. Phytother Res 24(Suppl 2): S168–S174. https://doi.org/10.1002/ptr.3060
Hoffmann DB, Griesel MH, Brockhusen B et al. (2016) Effects of 8-prenylnaringenin and whole-body vibration therapy on a rat model of osteopenia. J Nutr Metab. https://doi.org/10.1155/2016/6893137
Parfitt A, Drezner MK, Glorieux FH et al (1987) Bone histomorphometry: standardization of nomenclature, symbols, and units: report of the ASBMR Histomorphometry Nomenclature Committee. J Bone Miner Res 2(6):595–610. https://doi.org/10.1002/jbmr.5650020617
Bouxsein ML, Boyd SK, Christiansen BA et al (2010) Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J Bone Miner Res 25(7):1468–1486. https://doi.org/10.1002/jbmr.141
Sehmisch S, Komrakova M, Kottwitz L et al (2015) Effects of urocortin on spine? Osteologie 24(2):99–106
Komrakova M, Stuermer EK, Sturm A, Schmelz U, Tezval M, Stuermer KM, Sehmisch S (2012) Efficiency of 48 h vs. 24 h Injection of parathyroid hormone for amelioration of osteopenic spine properties in male rats. TOBONEJ 4(1): 20–26. https://doi.org/10.2174/1876525401204010020
Dachverband OeV (2014) Leitlinie des Dachverbands der Deutschsprachigen Wissenschaftlichen Osteologischen Gesellschaften e.V.: Prophylaxe, Diagnostik und Therapie der OSTEOPOROSE bei Männern ab dem 60. Lebensjahr und bei postmenopausalen Frauen. http://www.dv-osteologie.org/uploads/Leitlinie%202014/DVO-Leitlinie%20Osteoporose%202014%20Kurzfassung%20und%20Langfassung%20Version%201a%2012%2001%202016.pdf. Accessed 10 Dec 2017
Cortet B (2011) Use of strontium as a treatment method for osteoporosis. Curr Osteoporos Rep 9(1):25–30. https://doi.org/10.1007/s11914-010-0042-z
Iniguez-Ariza NM, Clarke BL (2015) Bone biology, signaling pathways, and therapeutic targets for osteoporosis. Maturitas 82(2):245–255. https://doi.org/10.1016/j.maturitas.2015.07.003
Abboskhujaeva LS, Ismailov SI, Alikhanova NM (2014) Efficacy of strontium ranelate in combination with a D-hormone analog for the treatment of postmenopausal osteoporosis. Drugs R D 14(4):315–324. https://doi.org/10.1007/s40268-014-0069-1
Nielsen SP, Slosman D, Sorensen OH et al (1999) Influence of strontium on bone mineral density and bone mineral content measurements by dual X-ray absorptiometry. J Clin Densitom 2(4):371–379
Reginster JY, Kaufman JM, Goemaere S et al (2012) Maintenance of antifracture efficacy over 10 years with strontium ranelate in postmenopausal osteoporosis. Osteoporos Int 23(3):1115–1122. https://doi.org/10.1007/s00198-011-1847-z
Hegde V, Jo JE, Andreopoulou P et al (2016) Effect of osteoporosis medications on fracture healing. Osteoporos Int 27(3):861–871. https://doi.org/10.1007/s00198-015-3331-7
Tarantino U, Celi M, Saturnino L et al (2010) Strontium Ranelate and bone healing: report of two cases. Clin Cases Miner Bone Metab 7(1):65–68
Fuchs RK, Allen MR, Condon KW et al (2008) Strontium ranelate does not stimulate bone formation in ovariectomized rats. Osteoporos Int 19(9):1331–1341. https://doi.org/10.1007/s00198-008-0602-6
Bain SD, Jerome C, Shen V et al (2009) Strontium ranelate improves bone strength in ovariectomized rat by positively influencing bone resistance determinants. Osteoporos Int 20(8):1417–1428. https://doi.org/10.1007/s00198-008-0815-8
Dahl SG, Allain P, Marie PJ et al (2001) Incorporation and distribution of strontium in bone. Bone 28(4):446–453
Deeks ED, Dhillon S (2010) Spotlight on strontium ranelate: in postmenopausal osteoporosis. Drugs Aging 27(9):771–773. https://doi.org/10.2165/11206440-000000000-00000
Body JJ, Bergmann P, Boonen S et al. (2012) Extraskeletal benefits and risks of calcium, vitamin D and anti-osteoporosis medications. Osteoporos Int (23 Suppl 1): S1–S23. https://doi.org/10.1007/s00198-011-1891-8
EMEA (2005) EPAR: scientific discussion: european medicines agency. http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Scientific_Discussion/human/000560/WC500045522.pdf. Accessed 10 Dec 2017
Tsai W-JA, McCormick KM, Brazeau DA et al (2007) Estrogen effects on skeletal muscle insulin-like growth factor 1 and myostatin in ovariectomized rats. Exp Biol Med 232(10):1314–1325. https://doi.org/10.3181/0704-RM-92
Velloso CP (2008) Regulation of muscle mass by growth hormone and IGF-I. Br J Pharmacol 154(3):557–568. https://doi.org/10.1038/bjp.2008.153
Kirk S, Oldham J, Kambadur R et al (2000) Myostatin regulation during skeletal muscle regeneration. J Cell Physiol 184(3):356–363
Roth SM, Walsh S (2004) Myostatin: a therapeutic target for skeletal muscle wasting. Curr Opin Clin Nutr Metab Care 7(3):259–263
Jasuja R, LeBrasseur NK (2014) Regenerating skeletal muscle in the face of aging and disease. Am J Phys Med Rehabil 93(11 Suppl 3):S88–S96. https://doi.org/10.1097/PHM.0000000000000118
Wagner PD (2011) The critical role of VEGF in skeletal muscle angiogenesis and blood flow. Biochem Soc Trans 39(6):1556–1559. https://doi.org/10.1042/BST20110646
Hoier B, Hellsten Y (2014) Exercise-induced capillary growth in human skeletal muscle and the dynamics of VEGF. Microcirculation 21(4):301–314. https://doi.org/10.1111/micc.12117
Ammann P, Badoud I, Barraud S et al (2007) Strontium ranelate treatment improves trabecular and cortical intrinsic bone tissue quality, a determinant of bone strength. J Bone Miner Res 22(9):1419–1425. https://doi.org/10.1359/jbmr.070607
Chen B, Li Y, Yang X et al (2013) Comparable effects of alendronate and strontium ranelate on femur in ovariectomized rats. Calcif Tissue Int 93(5):481–486. https://doi.org/10.1007/s00223-013-9765-z
Vegger JB, Bruel A, Sorensen TG et al (2016) Systemic Treatment with strontium ranelate does not influence the healing of femoral mid-shaft defects in rats. Calcif Tissue Int 98(2):206–214. https://doi.org/10.1007/s00223-015-0077-3
Hoffmann DB, Sehmisch S, Am, Hofmann et al (2017) Comparison of parathyroid hormone and strontium ranelate in combination with whole-body vibration in a rat model of osteoporosis. J Bone Miner Metab 35(1):31–39. https://doi.org/10.1007/s00774-016-0736-0
Boyd SK, Szabo E, Ammann P (2011) Increased bone strength is associated with improved bone microarchitecture in intact female rats treated with strontium ranelate: a finite element analysis study. Bone 48(5):1109–1116. https://doi.org/10.1016/j.bone.2011.01.004
Rizzoli R, Laroche M, Krieg MA et al (2010) Strontium ranelate and alendronate have differing effects on distal tibia bone microstructure in women with osteoporosis. Rheumatol Int 30(10):1341–1348. https://doi.org/10.1007/s00296-010-1542-y
Wu Y, Adeeb SM, Duke MJ et al (2013) Compositional and material properties of rat bone after bisphosphonate and/or strontium ranelate drug treatment. J Pharm Pharm Sci 16(1):52–64
Oliveira JP, Querido W, Caldas RJ et al (2012) Strontium is incorporated in different levels into bones and teeth of rats treated with strontium ranelate. Calcif Tissue Int 91(3):186–195. https://doi.org/10.1007/s00223-012-9625-2
Li C, Paris O, Siegel S et al (2010) Strontium is incorporated into mineral crystals only in newly formed bone during strontium ranelate treatment. J Bone Miner Res 25(5):968–975. https://doi.org/10.1359/jbmr.091038
Kalu DN (1991) The ovariectomized rat model of postmenopausal bone loss. Bone Miner 15(3):175–191
Acknowledgements
We wish to express our gratitude to Elsbeth Bonhoff Stiftung for providing the financial support for the present study (Grant N70). Moreover, we are grateful to R. Wigger, R. Castro-Machguth, and A. Witt for the technical support they provided.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors D. Saul, B. Harlas, A. Ahrabi, R. L. Kosinsky, D. B. Hoffmann, M. Wassmann, R. Wigger, K. O. Böker, S. Sehmisch, and M. Komrakova declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted.
Electronic supplementary material
Below is the link to the electronic supplementary material.
223_2017_374_MOESM1_ESM.eps
ATPase analysis of M. gastrocnemius, M. longissimus and M. soleus. area: cross sectional area of muscle fibers; dia: mean diameter of muscle fibers; oxi: oxidative and intermittent muscle fibers (I and IIa); gly: glycolytic muscle fibers (IIb). Data corrected by body weight (g). A,B, G-J,: Dunn´s test; C-F: Tukey-test. *p < 0.05, **p < 0.01, ***p < 0.001. (EPS 3448 KB)
223_2017_374_MOESM2_ESM.eps
Activity of lactate dehydrogenase (LDH), citrate synthase (CS) and Complex I was measured in M. gastrocnemius, M. longissimus and M. soleus. There were no significant differences between the groups (p > 0.05). A, H: Dunn´s test; B-G, I: Tukey-test (EPS 3226 KB)
Rights and permissions
About this article
Cite this article
Saul, D., Harlas, B., Ahrabi, A. et al. Effect of Strontium Ranelate on the Muscle and Vertebrae of Ovariectomized Rats. Calcif Tissue Int 102, 705–719 (2018). https://doi.org/10.1007/s00223-017-0374-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0374-0