Abstract
The activity of polyphenolic compounds, triterpenoids, carotenoids, chlorophylls and antioxidants in leaves and rhizomes of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) grown in Poland was investigated. Leaves and rhizomes were assessed for the presence of bioactive compounds with the ultra-performance liquid chromatography photodiode detector-quadrupole/time-of-flight mass spectrometry (UPLC-PDA-Q/TOF-MS) method, and for antioxidant activity with the on-line UPLC-ABTS screening. Forty-six polyphenolic compounds (15 phenolic acids, 12 flavones and flavonols, 11 flavan-3-ols and 8 stilbenes), were identified in Fallopia japonica and Fallopia sachalinensis. Furthermore, accurate mass measurement technique was for the first time in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) in leaves and rhizomes it identified 25 new compounds belonging to carotenoids (9), chlorophylls (13) and triterpenoids (3) as well as rated the antioxidant properties of each polyphenolic compound. Major qualitative differences were found in the profiles. The leaves and rhizomes were found to be a good source not only of (average 20408.18 and 2716.42 mg/100 g dm), but also chlorophylls (average 179.97 and 43.82 mg/100 g dm), carotenoids (average 100.23 and 53.25 mg/100 g dm) and triterpenoids (average 580.87 and 434.05 mg/100 g dm). The content of bioactive compounds in Fallopia japonica Houtt was around 8.0, 4.0, 2.0 and 1.3 times higher than the content of polyphenols, chlorophylls, carotenoids and triterpenoids in Fallopia sachalinensis (F.Schmidt). The accurate identification of Fallopia bioactive compounds is an indispensable detailed knowledge of the profile and step toward better understanding of the medicinal properties of the species and also potentially more extensive use of the plant.
Similar content being viewed by others
Introduction
Fallopia—Japanese knotweed—herbaceous perennial, strongly branching. Fallopia naturally occurs i.a. in Japan, the Kuril Islands, Sakhalin, Korea, South-West China, Taiwan, Vietnam [1]. It occurs there in river valleys, at the edge of forests and at roadside. Growing up to a height of 3–5 m, rhizomes produce substances that inhibit the growth of other plants. Green leaves, 5–15 cm long, broad in shape is the plant grows very fast—young stems can grow up to 10–15 cm per day. It has thick, wide, dark yellow rhizomes in cross-section, with reddish or brown bark [2,3,4].
In Korea and China, knotweed has been known for thousands of years as a medicinal plant [5, 6]. In addition, Japanese knotweed is a plant used in phototherapy [7]. In traditional Chinese and Japanese herbal medicine, this raw material is recommended as analgesic, antipyretic, diuretic and expectorant. It is used in the treatment of diseases, including asthma, atherosclerosis, cough, inflammation, hypertension, heart disease, fungal and bacterial infections and tumors [8]. Japanese knotweed also displays several beneficial biological effects such as inhibition of topoisomerases and neuraminidases, anti-oxidancy, anti-tumor activity, neuroprotective properties and inhibition of the development of borreliosis [3, 9,10,11]. Thanks to these properties, Japanese knotweed can be used as an alternative material of natural origin, which is a source of bioactive compounds in the prevention or treatment of many diseases.
Raw material obtained from the natural state and crops can be used in the pharmaceutical, cosmetic and food industry as well as in phototherapy. In order to better understand the potential of the plant and the possibility of its use, a thorough analysis of compounds with pro-health effects is necessary. Ultra-performance liquid chromatography coupled with a photodiode detector-quadrupole and tandem time-of-flight mass spectrometry (UPLC-PDA-Q/TOF-MS) is proved to be extremely useful for UV–Vis spectrum, peak assignment and further characterization of individual compounds. Particularly, the electrospray ionization mass spectrometry (ESI-MS) has been widely applied in the identification of phenolic compounds. In most instances, particular bioactive compounds can be identified directly by comparison with data reported in previous literature or authentic standards. Additionally, combination of separation and activity measurement, i.e., sensitive on-line HPLC-ABTS assays for analyzing free radical scavenging activity, exhibits a prominent advantage for screening and evaluating antioxidants particular bioactive compounds without loss of active components. Therefore, the methods are focused on the analyses of free radical scavenging activities of complex mixtures, especially the plant extracts [12, 13]. So far, to the best of our knowledge, there have been no reports regarding the properties of individual carotenoids, chlorophylls and triterpenoids as well as the antioxidant properties of each polyphenolic compound of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt), especially in leaves and rhizomes. Therefore, the aim of this study was to identify, quantify and compare a broad range of potential health-promoting components (polyphenols, carotenoids, chlorophylls, triterpenoids) by UPLC-PDA-Q/TOF-MS, and their on-line UPLC-ABTS assay in leaves and rhizomes of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) grown in Poland.
Materials and methods
Chemicals
Acetonitrile, formic acid, methanol, all-trans-β-carotene, all-trans-lutein, all-trans-zeaxanthin, violaxanthin, chlorophyll a, chlorophyll b, chlorophyllide b, pheophytin a, pheophytin b, betulinic, oleanolic and ursolic acid, ABTS 2,2′-azinobis(3-ethylbenzothiazoline-6-sulfonic acid), 6-hydroxy-2,5,7,8-tetramethylchroman-2-carboxylic acid (Trolox), 2,4,6-tri(2-pyridyl)-s-triazine (TPTZ), methanol acetic acid, and phloroglucinol were purchased from Sigma-Aldrich (Steinheim, Germany). (−)-Epicatechin, (+)-catechin, procyanidin B2,, caffeic acid, p-coumaric acid, 3-O-caffeoylquinic, 5-O-caffeoylquinic, ferulic acid, galloyl glucose, caftaric acid, luteolin 7-O-galactoside, apigenin 7-O-glucoside, kaempferol-3-O-galactoside, quercetin-3-O-rutinoside, quercetin-3-O-galactoside, quercetin-3-O-glucoside, quercetin-3-O-arabinoside, quercetin-3-O-xyloside, cis-piceid, trans-piceid, cis-resveratrol were purchased from Extrasynthese (Lyon, France). Acetonitrile for ultra-phase liquid chromatography (UPLC; Gradient grade) and ascorbic acid were purchased from Merck (Darmstadt, Germany).
Plant materials
Leaves and rhizomes of Fallopia japonica and Fallopia sachalinensis were used in the study. Material samples (~ 1.0 kg each) were collected from the Garden of Medicinal Plants herbarium at the Wroclaw Medical University, Poland. The fresh leaves and rhizomes were directly frozen at − 25 °C and then freeze-dried (24 h; Christ Alpha 1–4 LSC; Germany). The homogeneous dry material was obtained by crushing the dried tissues using a closed laboratory mill (IKA A.11, Germany). The powders were kept in a refrigerator (− 80 °C) until extract preparation.
Qualitative and quantitative assessment of polyphenols
The samples (1 g) were extracted with by 10 mL of mixture containing HPLC-grade methanol (30 mL/100 mL), ascorbic acid (2.0 g/100 mL) and acetic acid in an amount of 1.0 mL/100 mL of reagent. The extraction was performed twice by incubation for 20 min under sonication (Sonic 6D, Polsonic, Warsaw, Poland) and with occasional shaking. Next, the slurry was centrifuged at 19,000g for 10 min, and the supernatant was filtered through a Hydrophilic PTFE 0.20 µm membrane (Millex Samplicity Filter, Merck, Darmstadt, Germany) and used for analysis. The content of polyphenols in individual extracts was determined by means of the ultra-performance liquid chromatography-photodiode array detector–mass spectrometry method. All extractions were carried out in triplicate.
Qualitative (LC/MS Q-TOF) and quantitative (UPLC-PDA-FL) estimation of polyphenol (flavan-3-ols, flavonols, flavanone, stilbenes and phenolic acids) of Fallopia japonica and Fallopia sachalinensis extracts was carried out using an ACQUITY Ultra Performance LC system equipped with a photodiode array detector with a binary solvent manager (Waters Corporation, Milford, MA, USA) series with a mass detector G2 Q/TOF micro-mass spectrometer (Waters, Manchester, UK) equipped with an electrospray ionization (ESI) source operating in negative and positive modes. Separations of individual polyphenols were carried out using a UPLC BEH C18 column (1.7 mm, 2.1 × 100 mm, Waters Corporation, Milford, MA; USA) at 30 °C. The samples (10 µL) were injected, and the elution was completed in 15 min with a sequence of linear gradients and isocratic flow rates of 0.45 mL min−1. The mobile phase consisted of Solvent A (2.0% formic acid, v/v) and Solvent B (100% acetonitrile). The program began with isocratic elution with 99% Solvent A (0–1 min), and then a linear gradient was used until 12 min, reducing Solvent A to 0%; from 12.5 to 13.5 min, the gradient returned to the initial composition (99% A), and then, it was held constant to re-equilibrate the column. The analysis was carried out using full-scan, data-dependent MS scanning from m/z 100 to 1500. Leucine enkephalin was used as the reference compound at a concentration of 500 pg/L, at a flow rate of 2 L/min, and the [M–H]− ion at 554.2615 Da was detected. The [M–H]− ion was detected during a 15-min analysis performed within ESI-MS accurate mass experiments, which were permanently introduced via the Lock-Spray channel using a Hamilton pump. The lock mass correction was ± 1.000 for the mass window. The mass spectrometer was operated in negative and positive ion mode, set to the base peak intensity (BPI) chromatograms and scaled to 12,400 counts per second (cps) (100%). The optimized MS conditions were as follows: capillary voltage of 2500 V, cone voltage of 30 V, source temperature of 100 °C, desolvation temperature of 300 °C and desolvation gas (nitrogen) flow rate of 300 L/h. Collision-induced fragmentation experiments were performed using argon as the collision gas, with voltage ramping cycles from 0.3 to 2 V. Characterization of the single components was carried out via the retention time and the accurate molecular masses. Each compound was optimized to its estimated molecular mass in the negative mode, before and after fragmentation. The data obtained from UPLC–MS were subsequently entered into the MassLynx 4.0ChromaLynx Application Manager software (Waters). On the basis of these data, the software is able to scan different samples for the characterized substances. The runs were monitored at the following wavelengths: flavan-3-ols at 280 nm, phenolic acids at 320 nm, flavonol at 360 nm. The PDA spectra were measured over the wavelength range of 200–600 nm in steps of 2 nm. The retention times and spectra were compared to those of the authentic standards. The quantification of phenolic compounds was performed by external calibration curves, using reference compounds selected based on the principle of structure-related target analyte/standard (chemical structure or functional group).
The calibration curve for 3-caffeoylquinic acid was used to quantify quinic acid, caffeoylquinic acid isomers cis-3-O-caffeoylquinic acid, cis-5-O-caffeoylquinic acid, 3,4-di-O-caffeoylquinic acid and 4,5-di-O-caffeoylquinic acid. The calibration curve for caffeic acid was used to quantify caffeoyl glucoside. The calibration curve of p-coumaric acid was used, besides its own quantification, to quantify p-coumarylquinic acid. The calibration curve for ferulic acid was used to quantify feruloylquinic acid. Galloyl glucose, caftaric acid, 3-O-caffeoylquinic acid, 5-O-caffeoylquinic acid were quantified with their own standards. The calibration curve for procyanidin B2 was used to quantify all B-type procyanidins. The calibration curve for (+)-catechin was used to quantify (+)-catechin glucoside and (+)-catechin gallate. (+)-catechin and (−)-epicatechin were quantified with its own standard. The calibration curve for kaempferol 3-O-glucoside was used for its own quantification as well as kaempferol rhamnoside. The calibration curve for luteolin 7-O-galactoside was used for its own quantification as well as luteolin 7-O-glucoside. The calibration curve for apigenin 7-O-glucoside standard was used for its own quantification as well as apigenin 7-apiosylglucoside. The calibration curves of quercetin rutinoside, 3-O-glucoside and 3-O-galactoside were used to quantify quercetin derivatives. The calibration curves of Cis-piceid, trans-piceid, cis-resveratrol were used to quantify piceid and resveratrol derivatives. Cis-piceid, trans-piceid, cis-resveratrol were quantified with its own standard. All measurements were repeated three times. The results were expressed as mg/100 g of dry matter (dm).
Analysis of proanthocyanidins by phloroglucinolysis
Direct phloroglucinolysis of freeze–dried samples was performed as described by Lachowicz et al. [14] and Oszmiański and Lachowicz [15]. Materials lyophilisates were weighed in an amount of 5 mg in 2-mL Eppendorf vials. Subsequently, 0.8 mL of the methanolic solution of phloroglucinol (75 g/L) and ascorbic acid (15 g/L) were added to samples. After addition of 0.4 mL of methanolic HCl (0.3 M), the vials were incubated for 30 min at 50 °C with continuous vortexing in a thermo-shaker (TS-100, BioSan, Riga, Latvia). The reaction was terminated by placing the vials in an ice bath, drawing 0.6 mL of the reaction medium and diluting with 1.0 mL of sodium acetate buffer (0.2 M). The samples were centrifuged immediately at 20,000g for 10 min at 4 °C, and stored at 4 °C before reverse-phase HPLC (RP-HPLC) analysis. All incubations were done in triplicate. Phloroglucinolysis products were separated on a Cadenza CD C18 (75 mm × 4.6 mm, 3 µm) column (Imtakt, Japan). The liquid chromatograph was a Waters (Milford, MA) system equipped with diode array and scanning fluorescence detectors (Waters 474) and an autosampler (Waters 717 plus). Solvent A (25 mL aqueous acetic acid and 975 mL water) and solvent B (acetonitrile) were used in the following gradients: initial, 5% B; 0–15 min to 10% B linear; 15–25 min to 60% B linear; followed by washing and reconditioning of the column. Other parameters were as follows: a flow rate of 1 mL/min, an oven temperature of 15 °C, and volume of filtrate injected onto the HPLC system was 20 µL. The fluorescence detection was monitored at 278 nm and 360 nm. The calibration curves were established using (+)-catechin and (−)-epicatechinphloroglucinol adducts standards. All data were obtained in triplicate. The results were expressed as mg/100 g dm.
Identification and quantification of carotenoids and chlorophylls
For the extraction of carotenoids, a protocol similar to that described previously was applied [16]. The samples (0.5 g) containing 10% of MgCO3 were continuously shaken at 500 rpm (DOS-10L Digital Orbital Shaker, Elmi Ltd., Riga, Latvia) for 30 min in the dark with 5 mL of hexane:acetone:methanol (2:1:1, v/v/v) containing 1% BHT. After the first extraction, the samples were centrifuged at 19,000g for 10 min at 4 °C, and the supernatant was recovered. The samples were re-extracted and centrifuged in the same conditions. Supernatants were combined and evaporated to dryness. The pellet was re-extracted using 2 mL of 100% methanol, filtered through a hydrophilic PTFE 0.20_m membrane (Millex Samplicity Filter, Merck) and used for analysis.
Compounds were separated with an ACQUITY UPLC BEH RP C18 column (1.7_m, 2.1 mm × 100 mm,Waters Corp.) at 32 °C. The elution solvents were ACN : MeOH (7:3, v/v) (A) and 0.1% formic acid (B). Samples (10 µL) were eluted according to the linear gradient: 0–0.6 min, 25% B, 0.5 mL/min (isocratic); 0.6– 6.5 min, 4.9% B, 0.5 mL/min (linear gradient); 6.5–7.5 min, 0% B, 0.7 mL/min (linear gradient); 7.5–13.6 min, 0% B, 0.7 mL/ min (isocratic); 13.6–14.1 min, 25% B, 0.5 mL/min (linear gradient); and 14.1–16.6 min, 25% B, 0.5 mL/min (isocratic).Weak and strong needle solvents were ACN–MeOH (7:3, v/v) and 2-propanol, respectively.
Identification of carotenoids was carried out on the basis of fragmentation patterns and on the basis of PDA profiles. Where available, compounds were compared with authentic standards (their fragmentation pathways, retention times and PDA profiles). If standards were not available, fragmentation pathways and PDA profiles were compared with literature data. The runs were monitored at 450, 427 and 650 nm. The PDA spectra were measured over the wavelength range of 200–800 nm in steps of 2 nm. Calibration curves were made from all-trans-β-carotene, all-trans-zeaxanthin, all-trans-lutein, violaxanthin, neoxanthin, chlorophyll a, chlorophyll b, chlorophyllide b, pheophytin a, pheophytin b. (8′R) neochrome and (8′S) neochrome derivatives were expressed as neoxanthin. Hydrooxypheophytin a and b, pheophytin a′ and b′ were expressed as pheophytin a and b. Hydroxychlorophyll a and chlorophyll a′ and b′ were expressed as chlorophyll a and b.
All incubations were done in triplicate. The results were expressed as mg/100 g of dm.
Identification and quantification of triterpenoids
Sample extraction was performed as described by Farneti et al. [17]. The samples (0.5 g) were extracted with 5 mL of ethyl acetate and 5 mL of hexane. The extraction was performed by incubation for 20 min under sonication (Sonic 6D, Polsonic, Warsaw, Poland) with occasional shaking. After the first extraction, the samples were kept at 4 °C overnight. On the next day the samples were re-extracted in the same conditions. After the first extraction, the samples were centrifuged at 19,000g for 10 min at 4 °C, and the supernatant was recovered. The samples were re-extracted and centrifuged in the same conditions. Supernatants were combined and evaporated to dryness. The pellet was re-extracted using 2 mL of 100% methanol, filtered through a hydrophilic PTFE 0.20_m membrane (Millex Samplicity Filter, Merck) and used for analysis.
Identification and quantification of ursolic, oleanolic, and betulinic acids was done using the ACQUITY Ultra Performance LC system with a binary solvent manager (Waters Corp., Milford, MA, USA), a UPLC BEH C18 column (1.7 µm, 2.1 mm × 150 mm, Waters Corp., Milford, MA, USA), and a Q-TOF mass spectrometer (Waters, Manchester, UK) equipped with an electrospray ionization (ESI) source, operating in negative mode. The elution solvents were 100% methanol (A) and 100% acetonitrile (B) (15:85, v/v). Ursolic, oleanolic, and betulinic acids were eluted isocratically at a flow rate of 0.1 mL/min for 10 min at 20 °C. The m/z for betulinic acid was 455.34, for oleanolic acid 455.34, and for ursolic acid 455.33, and the retention times were 6.99, 7.66 and 8.36 min, respectively. The compounds were monitored at 210 nm. All data were obtained in triplicate. The results were expressed as mg/100 g of dm.
HPLC-PDA-on-line-ABTS-based assay
The antioxidant activity of individual HPLC peaks was measured using an on-line HPLC antioxidant detector system based on the TEAC assay of Re et al. [18] and Kusznierewicz et al. [19].
A CADENZA C18 column (75 mm × 4.6 mm i.d., 3 µm; Tokyo, Japan) with a C18 guard column was used. The column temperature was set at 30 °C. The separation was achieved by a gradient elution of 2.0% formic acid solution (solvent A) and acetonitrile (solvent B) at a flow rate of 0.6 mL/min: 0–30 min, 2–40% B; and up to 45 min column was recognition. The injection volume of sample was 10 µL, and the detection wavelength was set at 280 and 734 nm. ABTS radical cation was produced as described previously by Re at al [18]. After the first PDA detector, the mobile phase was mixed to the ABTS radical cation delivered by the second pump at a flow rate of 0.2 mL/min. The mixture was guided through a 25-m-long PTFE reaction coil with 0.25 mm internal diameter at 40 °C to a second UV detector, where ABTS decolourization was detected as a negative peak at 734 nm.
Statistical analysis
Statistical analysis, one-way ANOVA and hierarchal cluster (HA) and principal component analysis (PCA) were conducted using Statistica version 12.5 (StatSoft, Kraków, Poland). Significant differences (p ≤ 0.05) between mean values were evaluated by one-way ANOVA and Duncan’s multiple range test.
Results and discussion
Identification and quantification of phenolics in Fallopia
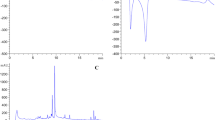
The identification and quantification of 46 compounds belonging to flavones and flavonols, phenolic acids, flavan-3-ols and stilbenes was based on a comparison with available standards of their MS, MS/MS data and retention times and literatures [20,21,22,23].The results concerning polyphenolic compounds analyzed by a UPLC-PDA-Q/TOF-MS system are summarized in Tables 1, 2, 3 and 4 and Fig. 1.
The main group of phenolics in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) leaves, which contained 37 compounds, were flavan-3-ols (monomers, oligomers and polymeric procyanidins) (~ 70%) > flavones and flavonols (~ 15%) > phenolic acid (~ 13%) ≥ stilbenes (~ 2%); however, in rhizomes, which contained 34 compounds, the main group were flavan-3-ols (~ 53%) > phenolic acid (~ 31%) ≥ flavones and flavonols (each ~ 8%). The leaves and rhizomes are good source of polyphenolic compounds and their average content in leaves was 20408.18 mg/100 g dm and was around 8.0 times higher than in rhizomes. The leaves and rhizomes of Fallopia japonica Houtt were more fertile around 1.5 and 1.2 times than anatomical parts of Fallopia sachalinensis (F.Schmidt). According to Shitasue et al. [24], the content of polyphenolics in Fallopia japonica grown in Hoshigaoka, Nagoya was around 5.0 and 7.0 times lower and 1.5 and 2.0 times higher than the content of these compounds in leaves and rhizomes of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) after ethanol extract. In comparison to other plants from the same family as Rumex japonicas and Rumex acetosa, the content of polyphenolic compounds was around 13 and 1.4 times lower and 4.8 times lower and 1.8 times higher than in leaves and rhizomes of Fallopia japonica Houtt [24]. The content of phenolics in leaves of Allium ursinum was around 15 and 18 times lower than the content of these compounds in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) [21]. The content of phenolics in leaves of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) was around 1.8 and 2.5 times higher than in Wild Rubus L. species [25]. Similarly, higher levels of phenolic compounds were presented in spinach leaves [26]. The content of individual compounds with antioxidant properties largely depends on factors such as variety, stage of maturity, part of the plant analyzed, climatic conditions, post-harvest handling and storage [26, 27].
Flavan-3-ols constituted a major group of the analyzed extracts and monomers, oligomers accounted for 13% and 20% but polymeric procyanidins accounted for 57% and 33% in leaves and rhizomes, respectively (Table 1). In this group, 11 flavan-3-ols were identified, of which 9 in leaves and 8 in rhizomes. Flavan-3-ols were detected in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) extracts: (+)-catechin and derivatives, (−)-epicatechin, 7 B-type procyanidin (dimer, tetramer, gallate).
The average content of flavan-3-ols (monomers and oligomers) and procyanidin polymers of Fallopia japonica Houtt were 1795.81 and 7070.04 mg/100 g dm and were 1.2 and 1.4 times higher than in Fallopia sachalinensis (F.Schmidt). The average content of flavan-3-ols in leaves of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) were 2835.37 mg/100 g dm of monomers and oligomers and 11290.10 mg/100 g dm of procyanidin polymer, which was around 6 and 10 times higher than in rhizomes (Table 1). The major compounds in flavan-3-ols (monomers and oligomers) were procyanidin type-B dimer and (+)-catechin glucoside. These compounds constituted an average of 22% and 18% of all flavan-3-ols. (+)-Catechin gallate in leaves and rhizomes constituted just 4% and 18%, but this compound is responsible for blocking the cell cycle of cancer in the G0/G1 phase by inhibiting topoisomerase I activity [28].
The next group belonging to polyphenols was flavones and flavonols. In this group, 12 compounds were detected in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt), of which 12 in leaves and 6 in rhizomes (Table 2).
The average content of flavones and flavonols in Fallopia japonica Houtt was 1911.07 mg/100 g dm and was 2.0 times higher than in Fallopia sachalinensis (F.Schmidt). The average content of flavones and flavonols in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) leaves amounted to 2989.68 mg/100 g dm and the content was around 19 times higher than in rhizomes (Table 2). Quercetin rhamnoside and pentoside were the major components of Fallopia japonica Houtt leaves and rhizomes extracts (62–72% and 12%, respectively, of all flavones and flavonols). The content of flavones and flavonols in leaves of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) was around 1.7 and 2.2 times lower than in Wild Rubus L. Species [25]. Similarly, higher levels of phenolic compounds were presented in spinach leaves [26, 29].
The second group belonging to polyphenolic compounds were phenolic acids. In this group, 15 compounds were detected in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt), of which 9 in leaves and 13 in rhizomes (Table 3).
The average content of phenolic acids in Fallopia japonica Houtt was 1898.47 mg/100 g dm and was 1.2 times higher than in Fallopia sachalinensis (F.Schmidt). The average content of phenolic acids in leaves of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) was 2927.97 mg/100 g dm and it was around 4 times higher than in rhizomes (Table 3). Caftaric and 5-O-caffeoylquinic acids were the major components in Fallopia japonica Houtt leaves and rhizomes extracts (34–7% and 25–47%, respectively, of all phenolic acids). The results regarding phenolic acids in leaves of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) were similar to the results in Wild Rubus L. Species [25].
The last group of phenolic compounds was stilbenes. In this group, 8 compounds were detected in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt), of which 7 in leaves and rhizomes (Table 4).
The average content of stilbenes in Fallopia japonica Houtt was 303.81 mg/100 g dm and was 1.2 times higher than in Fallopia sachalinensis (F.Schmidt). The average content of stilbenes in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) leaves amounted to 329.24 mg/100 g dm and it was around 2.0 times higher than in rhizomes (Table 4). Trans-piceid was the major component in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) leaves and rhizomes extracts (34–50% of all stilbenes). The leaves and rhizomes of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) are an excellent source of resveratrol and piceid. Similar results regarding resveratrol and piceid in Japanese knotweed leaves and rhizomes were presented by Beˇnova et al. [22]. According to Surguladze et al. [30], the content of resveratrol and piceid identified in red grape wine was around 24.0 and 7.0 times lower than in leaves and 9.0 and 5.0 times lower than in rhizomes of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt). Additionally, the content of piceid in grape cv. Casteao from Portugal was around 15.0 times lower than in leaves and rhizomes of Fallopia japonica Houtt and 16.0 times of Fallopia sachalinensis (F.Schmidt) [31, 32].
HPLC-UV-ABTS chromatograms (according to the formed negative inactivated ABTS•+ radical cation peaks) of the Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) rhizome extracts revealed that flavan-3-ols derivatives such as monomers and oligomers (Fig. 1, compounds no. 8, 9, 11, 14, 32 and 35) and trans-piceid and trans resveratrol (compounds 22 and 29) possess greater radical scavenging capacity than phenolic acids (Fig. 1, compounds no. 10, 12, 13, 16). Flavan-3-ols as catechins and procyanidins have two ortho-dihydroxy functional groups, which determine high antioxidant activity of these compounds. Additionally, Fig. 1 revealed that especially: (+)-catechin, procyanidin dimer B and trans-resveratroloside possess the strongest radical scavenging capacity. Furthermore, the on-line HPLC-ABTS assay used enables to evaluate the quality of materials and choice of species rich in antioxidants. The test allows to determine the antioxidant properties of unknown compounds, the precise antiradical activity of known compounds and effects on total antioxidant capacity of a medicinal preparation or medicinal herbal raw material [13, 29, 33,34,35].
Identification and quantification of chlorophylls and carotenoids
The results regarding carotenoids and chlorophylls analyzed by the UPLC-PDA-Q/TOF-MS system are summarized in Tables 5 and 6. Twenty-two compounds, of which 13 belong to chlorophylls and nine to carotenoids, were identified in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) of leaves and rhizomes. There were twice as many chlorophylls in the analyzed leaves but 1.5 times more carotenoids in rhizomes. These compounds have not been found in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) so far.
The average content of chlorophylls in Fallopia japonica Houtt was 135.64 mg/100 g dm and was 1.6 times higher than in Fallopia sachalinensis (F.Schmidt). The average amount of chlorophylls in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) of leaves was 179.97 mg/100 g dm and it was 4 times higher than in rhizomes (Table 5). The major compounds in the analyzed extracts were pheophytin b, chlorophyll a and b (~ 34–46%, 29–5% and 11–27% of all compounds). The same results were obtained in wild garlic leaves by Lachowicz et al. [23] and Moringa oleifera leaves by Sreelatha et al. [36]. According to Lachowicz et al. [23], the presented content of chlorophylls in wild garlic leaves was around 2.6 times higher than in Fallopia japonica Houtt leaves. In broccoli or guava, the content of chlorophylls was 1.7 and 6.0 and 1.4 and 4.9 times lower than in leaves of Fallopia japonica Houtt Fallopia sachalinensis (F.Schmidt) [37, 38].
The average content of carotenoids in Fallopia japonica Houtt was 93.02 mg/100 g dm and was 1.6 times higher than in Fallopia sachalinensis (F.Schmidt). The average content of carotenoid in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) leaves was 100.23 mg/100 g dm which was 2.0 times higher than in rhizomes. The major compounds in the analyzed extracts were all-trans-β-carotene and all-trans-lutein (~ 52–76% and 20–8% of all carotenoids). The same results were obtained in wild garlic by Lachowicz et al. [23] and Zea mays by Drążkiewicz et al. [39] In the leaves of Allium ursinum, the content of carotenoids was 5.0 and 3.9 times higher than in the leaves of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) [23], but the content of carotenoids in broccoli and coriander leaves was around 2.5 and 1.3 times and 1.4 and 1.1 times lower than in leaves of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) [40]. The content of carotenoids in Moringa oleifera leaves was 1.4 and 1.1 times lower than the content of these compounds in leaves of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) [37].
Identification and quantification of triterpenoid compounds
The results of identification and quantification of triterpenoids in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) (leaves and rhizomes) are presented in Table 7. Three triterpenoids were identified as betulinic (Rt = 6.99 min), ursolic (Rt = 7.66 min) and oleanolic acids (Rt = 8.36 min). The value of their molecular ion [M–H]− was m/z 455.3 [23]. Triterpenoids have not been found in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) so far.
The average content of triterpenoids in Fallopia japonica Houtt was 573.70 mg/100 g dm and was 1.3 times higher than in Fallopia sachalinensis (F.Schmidt). The average content of triterpenoids in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) leaves amounted to 580.87 mg/100 g dm and it was 1,5 times higher than in rhizomes. The major compound in leaves was ursolic acid (~ 58% of all triterpenoids), followed by oleanolic and betulinic acid (~ 29 and 13%). Similarly, the results regarding individual triterpenoids were presented in Olea europaea leaves [41]. In rhizomes, the major compound was oleanolic acid (~ 64%) and betulinic and oleanolic acids constituted 22% and 14%. According to Lachowicz et al. [23], the content of triterpenoids, namely betulinic, ursolic and oleanolic acids in wild garlic leaves was, respectively, 1.2 and 7.4 times lower and 1.2 times higher than in leaves of Fallopia japonica Houtt. In Allium ursinum, the major compound was oleanolic acid as it was in rhizomes of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt). The triterpenoids are dependent on several factors, such as environmental conditions, climate and degree of fruit maturity [41].
Cluster analysis
Cluster analysis (HCA) is a data analysis method, meaning that prior knowledge of the sample is not required. Cluster analysis enables interpretation of the results in a fairly intuitive, graphic way. HCA of bioactive compounds such as the polyphenols, carotenoids, chlorophylls, triterpenoids analyzed in samples, was used as an additional tool to assess heterogeneity among leaves (B) and rhizomes (A) of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) grown in Poland. Generally, cluster analysis presented three clear similarity clusters (Fig. 2). The highest correlation of bioactive compounds from rhizomes was obtained for polymeric procyanidins and total phenolic compounds. The lowest concentration was obtained for phenolic acids and flavan-3-ols (monomeric and oligomeric). The difference between the rhizomes and leaves was that the leaves were richer in flavan-3-ols (monomeric and oligomeric) and contained a lower concentration of stilbenes and flavones and flavonols.
Principal component analysis (PCA)
The differences between anatomical parts of Fallopia japonica (Houtt.) and Fallopia sachalinensis (F.Schmidt) species in their polyphenolic profiles and antioxidant properties were emphasized during principal component analysis (PCA). Two major PCs for the researched anatomical parts of species Fallopia japonica (Houtt.) and Fallopia sachalinensis (F.Schmidt) for 96.70% of total variability: for PC1 81.63%, and for PC2 15.03% (Fig. 3).
PCA mean showing the relationship among phenols, carotenoids, chlorophylls, triterpenoids in leaves and rhizomes of Fallopia japonica (FJ) and Fallopia sachalinensis (FS). UA, ursolic acid; OA, oleanolic acid; BA, betulinic acid; TF, total flavonols; PA, phenolic acid; F3O, flavan-3-ols; PP, polymeric procyanidins; TS, total stilbenes; (+)-C, (+)-Catechin; (−)-E, (−)-Epicatechin; PB, procyanidin B; Lu, luteolin; Ap, apigenin; Qu, quercetin; Ka, kaempferol; GG, galloyl glucose; Ca, caffeoylquinic; Co, coumaroylquinic; Fe, feruloylquinic; Pi, piceid; Re, resveratrol; TCC, total carotenoid compounds; Lu, lutein; Ze, zeaxanthin; Vi, violaxanthin; Ca, carotene; Ne, neochrome; TCh, total chlorophyll compounds; Chl, chlorophyllide; Ch, chlorophyll; Ph, pheophytin; TTC, total triterpenoid compounds
PC1 indicated the differences between the concentrations of flavan-3-ols (monomers and oligomers), polymeric procyanidins, flavonols, phenolic acids, stilbenes, total triterpenoids, betulinic, oleanolic, ursolic acid, total carotenoids and chlorophylls and their derivatives. PC2 showed the comparison of feruloylquinic acid. The research results showed some differences between the species Fallopia japonica (Houtt.) and Fallopia sachalinensis (F.Schmidt) and their leaves and rhizomes. For example, rhizomes of Fallopia sachalinensis (F.Schmidt) showed the highest feruloylquinic acid. Leaves of Fallopia japonica (Houtt.) and Fallopia sachalinensis (F.Schmidt) demonstrated higher concentrations of total bioactive compounds and their derivatives. Figure 3 shows that rhizomes of Fallopia japonica (Houtt.) showed the lowest content of analyzed parameters.
Conclusion
The results presented the significant effect of the measured anatomical parts of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) on the composition of bioactive compounds. In this study, 71 potential health-promoting compounds were identified, including for the first time 25 belonging to chlorophylls (13 compounds), carotenoids (nine compounds) and triterpenoids (three compounds) in Fallopia using the UPLC-PDA-Q/TOF-MS method. The leaves and rhizomes of Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) were found to be a good source not only of phenolics (average 20408.18 and 2716.42 mg/100 g dm), but also chlorophylls (average 179.97 and 43.82 mg/100 g dm), carotenoids (average 100.23 and 53.25 mg/100 g dm) and triterpenoids (average 580.87 and 434.05 mg/100 g dm). The content of bioactive compounds in Fallopia japonica Houtt was around 8.0, 4.0, 2.0 and 1.3 times higher than the content of polyphenols, chlorophylls, carotenoids and triterpenoids in Fallopia sachalinensis (F.Schmidt). Used HPLC-UV-ABTS chromatograms of the Fallopia japonica Houtt and Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) rhizomes extracts revealed that (+)-catechin, procyanidin dimer B, Cis 5-O-caffeoylquinic, 3-O-p-coumaroylquinic acids and trans-resveratroloside possess the strongest radical scavenging capacity. Furthermore, the on-line HPLC-ABTS assay used enabled to evaluate the quality of materials and demonstrated the differences between the leaves and the rhizomes rich in antioxidants. The study showed that Fallopia japonica Houtt leaves and rhizomes can be considered a significant source of bioactive components. Overall, Fallopia japonica Houtt leaves and rhizomes can be an excellent source of individual bioactive compounds showing a broad spectrum of biological activity. This raw material will certainly an interesting product to use in the pharmaceutical, cosmetic and food industry as well as in phototherapy (as a medicinal preparation or medicinal herbal raw material). However, it is necessary to constantly monitor and collect information on the spread of the species.
References
Alberternst B (2006) Online database of the North European and Baltic Network on Invasive Alien Species-NOBANIS
Bailey JP, Bimova K, Mandak B (2009) Biol Invas 11:1189–1203
Lee G, Choi TW, Kim C, Nam D, Lee SG, Jang HJ, Lee JH, Um JY, Jung SH, Shim BS, Ahn KS, Ahn KS (2012) Immunotoxicol 34:454–464
Peng W, Qin R, Li X, Zhou H (2013) J Ethnopharmacol 148(3):729–745
Jeong ET, Jin MH, Kim MS, Chang YH, Park SG (2010) Arch Pharmacal Res 33(9):1331–1338
Piroznikow E (2012) Pol Enthobiol 2:27–32
Nosalova G, Jurecek L, Hromadkova Z, Kostalova Z, Sadlonova V (2013) Neurobiol Respir 2013:51–57
Zhang H, Li C, Kwok ST, Zhang QW, Chan SW, Evid (2013) Based Complement Alternat Med 2013:208–349
Eid SY, El-Readi MZ, Ashour ML, Wink M, Evid (2015) Based Complement Alternat Med 2015:868424
Lee CC, Chen YT, Chiu CC, Liao WT, Liu YC, David HM (2015) J Biosci Bioeng 119:464–469
Xiao HT, Qi XL, Liang Y, Lin CY, Wang X, Guan ZZ, Hao XY (2014) Pharm Biol 52(3):356–361
Lin LZ, Harnly JM (2008) J Agric Food Chem 56(21):10105–10114
He W, Liu X, Xu H, Gong Y, Yuan F, Gao Y (2010) Food Chem 123(2):521–528
Lachowicz S, Oszmiański J, Pluta S (2017) Food Chem 235:234–243
Oszmiański J, Lachowicz S (2006) Molecules 21(8):1098
Delpino-Rius A, Eras J, Marsol-Vall A, Vilaró F, Balcells M, Canela-Garayoa R (2014) J Chromatogr A 1331:90–99
Farneti B, Masuero D, Costa F, Magnago P, Malnoy M, Costa G, Vrhovsek U, Mattivi F (2015) J Agric Food Chem 63(10):2750–2759
Re R, Pellegrini N, Proteggente A, Pannala A, Yang M, Rice-Evans C (1999) Free Rad Biol Med 26:1231–1237
Kusznierewicz B, Piasek A, Bartoszek A, Namiesnik J (2011) Phytochem Anal 22:392–402
Fan P, Hay AE, Marston A, Lou H, Hostettmann K (2009) Biochem System Eco 37(1):24–34
Ares AM, Soto ME, Nozal MJ, Bernal JL, Higes M, Bernal J (2015) Food Anal Method 8(6):1565–1575
Beňová B, Adam M, Pavlíková P, Fischer J (2010) J Supercritic Fluid 51(3):325–330
Lachowicz S, Kolniak-Ostek J, Oszmiański J, Wiśniewski R (2017) J Food Proces Preserv 41(1):e13089
Shitasue S, Hashimoto A, Mori Y, Mase T, Isshiki S (2016) J Sugiyama Jyogakuen 47
Oszmiański J, Wojdyło A, Nowicka P, Teleszko M, Cebulak T, Wolanin M (2018) Molecule 20(3):4951–4966
Suzuki K, Kamimura A, Hooker SB (2015) Mar Chem 176:96–109
Glavnik V, Vovk I, Albreht A (2017) J Chromatograph A 1482:97–108
Gan RY, Li HB, Sui ZQ, Corke H (2018) Critic Rev Food Sci Nutr 58(6):924–941
Slimestad R, Torskangerpoll K, Nateland HS, Johannessen T, Giske NH (2005) J Food Comp Anal 18(1):61–68
Slavin J, Marquart L, Jacobs Jr D (2000) Cereal Foods World 45(2):54–58
Surguladze MA, Bezhuashvili MG (2017) Ann Agrarian Sci 15(1):137–140
Romero-Pérez AI, Ibern-Gómez M, Lamuela-Raventós RM, de la Torre-Boronat MC (1999) J Agric Food Chem 47(4):1533–1536
Landis-Piwowar KR, Milacic V, Chen D, Yang H, Zhao Y, Chan TH, Big Y, Dou QP (2006) Drug Resis Updat 9(6):263–273
Marambaud P, Zhao H, Davies P (2005) J Biol Chem 280(45):37377–37382
Milenković SM, Zvezdanović JB, Anđelković TD, Marković DZ (2012) Adv Technol 1(1):16–24
Sreelatha S, Padma PR (2009) Plant Foods Hum Nutr 64(4):303
Delgado-Pelayo R, Gallardo-Guerrero L, Hornero-Méndez D (2014) Food Res Intern 65:272–281
Drążkiewicz M, Baszyński T (2005) J Plant Physiol 162(9):1013–1021
Saini RK, Nile SH, Park SW (2015) Food Res Intern 76:735–750
Somova LI, Shode FO, Ramnanan P, Nadar A (2003) J Ethnopharmacol 84(2–3):299–305
Loza-Mejía MA, Salazar JR (2015) Molecular 62:18–25
Acknowledgements
The publication was supported by Wroclaw Centre of Biotechnology, under the program The Leading National Research Centre (KNOW) for the years 2014–2018.
Author information
Authors and Affiliations
Contributions
Important contributions to the design and preparation of the manuscript: SL and JO. Contributions to sample and analysis experiments: SL and JO. Analysis of the experiment data: SL, JO, AW and TC. Critical revision of the important intellectual content: SL, JO, LH and MS. All authors contributed to the preparation of the paper and approved the final version.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Lachowicz, S., Oszmiański, J., Wojdyło, A. et al. UPLC-PDA-Q/TOF-MS identification of bioactive compounds and on-line UPLC-ABTS assay in Fallopia japonica Houtt and Fallopia sachalinensis (F.Schmidt) leaves and rhizomes grown in Poland. Eur Food Res Technol 245, 691–706 (2019). https://doi.org/10.1007/s00217-018-3191-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3191-4