Abstract
Changes in the biophysical traits of the pectoral muscles of chickens with deep pectoral myopathy (DPM) were analysed with selected instrumental techniques. For comparative purposes, the research used both samples of pectoralis minor muscles of 39–42-day-old Cobb 500 broiler chickens and pectoralis major muscles with DPM symptoms, as well. Computer Image Analysis (CIA) revealed that the pectoral minor muscles with DPM were characterised by smaller area of muscle fibres’ cross-sectional area (CSA) than normal muscles. A longitudinal section of DPM muscles also confirmed the presence of large spaces between the bundles of muscle fibres. The results of Differential Scanning Calorimetry (DSC) analysis showed that all the temperatures of transformations referring to minor muscles with myopathy symptoms were significantly lower than the temperatures noted in the muscles without DPM. The enthalpy values observed in both pectoral muscles with DPM were significantly lower than the values noted in healthy muscles. The water dynamics analysed by low-field NMR showed that the values of spin–lattice relaxation time T1 in p. major muscles without DPM were higher than the values in the muscles with the defect. On the other hand, an inverse dependence was observed in p. minor muscles samples. The value of the long relaxation time T22 was reduced in both muscles with pathological lesions. In conclusion, three advanced analytical tools used in this study (CIA, DSC, and LF NMR) provide new insight into the characterization of protein changes observed in the pectoral muscles of chicken broilers with DPM.
Similar content being viewed by others
Introduction
Poultry meat (mainly chicken meat) is the main driving force of the growth in total meat production due to the growing global demand for this animal protein, which is more affordable than red meats [1]. In the last 30 years, the dressed weight of broiler chickens has doubled (the live weight has increased by 30.2 g per year), and at the same time, the feed conversion rate (FCR) has decreased yearly by around 0.036% [2], whereas the breeding time has been reduced by half. Economic indicators rather than the birds’ natural behaviour were the priority. This policy caused numerous degenerations, especially in the birds’ pectoral muscles.
The poultry muscular system is not exempt from pathology and any condition which affects the quality of breast meat is of great importance to breeding companies and broiler producers. Carcasses affected by breast muscle myopathies (BMM) can be downgraded or in some cases condemned, resulting in economic losses for poultry meat producers [3, 4]. Bailey et al. [5] indicated three BMM types: deep pectoral myopathy (DPM; binary trait) as one of the earliest myopathies reported in poultry (turkeys, chickens), ‘white striping’ (WS; four categories), and ‘wooden breast’ (WB; three categories). Deep pectoral myopathy is chiefly observed in the pectoralis minor muscle, which is known as m. pectoralis supracoracoideus. In the recent years, researchers have observed higher intensity of the symptoms of DPM in specialised, high-efficiency muscular chickens of Cobb, Ross, and Flex genetic lines in Europe and the US [6, 7]. DPM lesions are initiated, while birds are still alive. Muscular necrosis occurs as a result of hypoxia and muscle malnutrition, during birds’ greater physical activity, especially when they flap their wings due to stress experienced during breeding [8, 9]. The specific location of the pectoralis minor muscle in a confined space, where relaxation is limited, plays a significant role in the aetiology of the abnormality. Contraction of the major pectoral muscles and the minor pectoral muscles are responsible for the down- and up-strokes of the wings. In an intensive breeding system, the birds’ physical activity is low. During exercise, a properly functioning smaller pectoral muscle increases its volume by even 25% [8]. Due to the inelastic fascia surrounding the muscle and its tight location next to the sternum, the pectoralis minor is unable to expand with the influx of blood during exertion. The increased size of the muscle is so marked in the heavy breeds that muscle becomes strangulated and ischemic, because the increased pressure within the muscle occludes the blood vessels and causes necrosis of the muscle [10].
The breast muscles of broiler chickens are made up of white (glycolytic and anaerobic) muscle fibres, which are extremely susceptible to ischaemic stress. The loss of cellular homeostasis [3] and breakdown of muscle fibre membrane integrity triggers a rapid inflammatory and regenerative response in the muscle. Following the ischaemia, there is rapid necrosis of the tissues and red blood cells in the muscle, giving rise to haemorrhaging and eventually greenish discolouration of the muscle [6]. Breast muscles affected in this way can exhibit localised or diffuse changes in organoleptic quality, e.g., colour, texture, composition [10, 11], and muscle structure [12]. It is noteworthy that the unfavourable increase in muscle hardness caused by advanced necrosis can be observed not only in deep pectoral muscles but also in superficial ones. They are less tender, more rubbery, and less elastic than pectoral muscles without DPM [7].
There have been a few studies using advanced instrumental techniques to investigate the mechanism of lesions in tissue with muscular dystrophy. It is possible to determine the functional characteristics of muscle proteins only by physical analyses, which are sufficiently sensitive to follow changes in intermolecular interactions taking place in the systems under investigation. They should supply information on molecular mechanisms in protein systems, simultaneously preserving the microstructure of the biopolymer system.
The recent development of computer technologies and colour image processing techniques has made the histological characterization of muscles by computer image analysis (CIA) more efficient. Imaging systems have been recommended also as a new tool for the measurement of poultry meat quality. In the past 20 years, the vision systems supported by cameras, computers, and software programs have been applied to characterise various muscle quality issues including the detection of processed carcass defects [13].
Differential scanning calorimetry (DSC) is a very useful tool for studying the thermal properties of proteins, especially in terms of the denaturation process. When a sample rich in proteins is heated at a programmed heating rate, endothermic peaks appear, which can be used as protein stability indicators. This technique enables the investigation of protein behaviour in situ in very small amounts. DSC has been widely used to analyse the muscle proteins from chicken, beef, pork [14,15,16], fish meat, and other freshwater sources [17, 18].
The nuclear magnetic resonance (NMR) method in low magnetic field describes, by use of relaxation time measurements, the quantitatively and qualitatively free water fraction, and fraction of water bound in the structure. This has been used in the research on the pectoral muscles of wooden breast chickens [19]. However, the pectoral muscles of chickens with myopathy have not been studied with this method.
Deep pectoral myopathy (DPM) primarily affecting smaller pectoral muscles is observed in gallinaceous poultry, i.e., turkey and chicken, and less frequently and to a lesser extent, in the major superficial muscles. Changes in muscle structure and gene expression pathways in p. major muscle of fast- and slow-growing commercial broilers with DPM were mentioned recently by Yalcin et al. [12]. Doubtless and because of the aesthetic appearance of the meat with intense green colour, DPM minor pectoral muscles are unfit for human consumption, although the technological usability of muscles with first DPM stage surface symptoms still seems to be interesting and it is worth considering as comparative research material. Because the lesion does not impair the general health of the chickens and this meat defect is not associated with any infectious or harmful substances, Kijowski and Kupinska [20] reported the possibility to process such breast meat using, i.e., curing and/or smoking treatments. Based on this approach, it seems reasonable to compare both major and minor pectoral muscle samples with DPM symptoms, as well as to use advanced analytical techniques with a general scope to extend knowledge of protein changes in further processed products.
Therefore, the aim of the study was to conduct a detailed analysis of the physical traits of pectoral muscles of chickens with DPM and to compare them with healthy muscles by means of several techniques applied at the same time. The pectoralis major and minor muscles were instrumentally assessed using the following techniques: computer image analysis of the muscle structure (CIA), differential scanning calorimetry (DSC), and low-field nuclear magnetic resonance (LF NMR).
Materials and methods
Materials
Pectoral muscles from broiler chickens of Cobb 500 genetic line, aged 39–42 days, and weighing 1.99–2.60 kg were used as the research material. The samples were collected in the local poultry slaughterhouse. Among the muscles demonstrating any DPM symptoms that were rejected during a typical work shift a total of eight breast samples with typical third-stage myopathy were identified and proposed for further testing. Details about the position of the collection of samples from the breast muscles of the chicken can be seen in Fig. 1. The muscles were stored for 24 h at a temperature of 3–4 °C and then samples were taken for further studies. In each case, measurements were conducted on pectoralis major and minor muscles, which had been cut out both from healthy poultry carcasses and from carcasses with symptoms of third-stage myopathy, i.e., with an intense green colour of the muscle tissue [4]. Samples of muscles collected for analysis were marked as follows:
A—M. pectoralis major—normal;
B—M. pectoralis minor—normal;
C—M. pectoralis major—DPM;
D—M. pectoralis minor—DPM;
Preparation of histological specimens
Cuboids measuring 10 × 10 × 30 (mm) were cut out from the collected muscle samples: A, B, C, D. Next, they were fixed in neutralised formalin to prevent tissue destruction. Fixed muscle samples were embedded in paraffin and cut into 10 µm slices, which were placed onto micro slide glass, dried, dewaxed, and prepared for staining. A combined slice staining was used applying Delafield haematoxylin and eosin. Histological specimens stained with Delafield and eosin were prepared from fresh meat.
Computer image analysis
The meat tissue structure was investigated by means of image analysis. An image taken with an Axiolab microscope using an OPTA-TECH HDMI camera (Warsaw, Poland) was transferred to a computer. Next, it was analysed with the MultiScan v.13.01 program. A uniform procedure of object identification and analysis was developed for all the preparations. The preparation structure was studied at constant microscope magnification (×200) and ten fields of constant area were analysed from each preparation. Fibres were identified, counted, and expressed as a percentage of the number of fibres per field. The fibre cross-sectional area (CSA) and fibre circumference were determined in each analysed preparation.
DSC thermodynamic analysis
Measurements were made with a Perkin Elmer DSC 7 device (Perkin Elmer, Norwalk) equipped with a Perkin Elmer Intra cooler II and Pyris software. Nitrogen (99.999% purity) was used as the purge gas. The DSC calorimeter was calibrated for temperature and enthalpy using indium (m.p. 156.6 °C, ∆Hf=28.45 J g− 1) and n-dodecane (m.p. − 9.65 °C, ∆Hf=216.73 J g− 1). The muscle samples were weighed, placed in 20 µL aluminium sample pans (Perkin Elmer, No. 0219-0062), and sealed. At least three samples were heated from 5 to 100 °C at a scanning rate of 10 °C min− 1 with a sealed empty pan as reference. The peak temperatures (T1, T2, and T3) and the peak heights for the first (ΔY1) and fifth (ΔY5) peak were recorded. The transition enthalpy (ΔH) was calculated from the peak area using the Pyris software and expressed as J per 1 g of sample material.
LF NMR measurements
Relaxation times were measured by use of a pulsed NMR spectrometer operating at a frequency of 15 MHz (ELLAB, Poznań, Poland). The inversion-recovery (\(\pi - \tau - \pi /2\)) impulse sequence [21] was applied for measurements of the T1 relaxation times. Calculations of the spin–lattice relaxation time values were performed with the assistance of the CracSpin program [22]. Mono-exponential magnetisation recovery was noted in all the systems under analysis. This means that the system was relaxed at one spin–lattice relaxation time T1. Measurements of the T2 spin–spin relaxation times were taken using the pulse train of the Carr–Purcell–Meiboom–Gill spin echoes \(\left( {\pi /2 - {\text{TE}}/2 - {{(\pi )}_n}} \right)\) [24].
The analysis revealed the presence of two proton fractions in all the systems, but the fast chemical exchanges were observed between free and bound water fraction [23]. The temperature of NMR was under control and amounted to 20.0 ± 0.5 °C.
Statistical analysis
Histological tests, DSC measurements, and low-NMR analysis were replicated four times for samples of both (major and minor) breast muscles. The significance (LSD test) of the results at p < 0.05 was tested by ANOVA (one way) analysis. All the results were analysed statistically using the software SPSS ver. 13.0 (SPSS Inc., USA).
Results and discussion
Computer image analysis
The intensity of DPM depends on many factors. Pectoral muscles affected by DPM exhibit degenerative necrotic lesions, i.e., Zenker’s necrosis. There are inflammatory cells, chiefly macrophages and heterophilic leucocytes [4]. Depending on the stage of the disease, necrotic muscle fibres begin to appear. They become drier and are surrounded by a layer of connective tissue, which separates them from the remaining part of the muscle tissue. Breast muscles that are affected show histological lesions typical of focal or diffuse ischaemia, including varying severities of muscle fibre fragmentation, swelling, degeneration as well as connective tissue, fat, and inflammatory cell infiltration [12]. Lesions in muscle fibres are also caused by the fact that pectoral muscles are chiefly composed of white muscle fibres, which acquire energy by anaerobic respiration, i.e., glycolysis. Glycogen is the principal energy substrate for this type of fibre and is metabolised into lactic acid, which is normally removed by the blood. Type 2 muscle fibres naturally produce energy aerobically through the vast majority of the animal’s life, but when energy levels need to increase (flapping and flying), the metabolism turns to producing energy the glycogenolytic way, although this only for a short period of time. After slaughtering, the large meat glycogen stores within the breast may cause a defect in the PSE type [10]. Berri et al. [24] studied the structural and metabolic characteristics of the pectoralis major muscle in relation to breast muscle fibre development in broiler chickens. The researchers showed that breast muscle weight and yield were positively correlated with muscle fibre diameter and negatively with glycogen levels. Studies on the myodegenerative problems that affect the muscles of the modern broiler point towards the rapid growth rate, as well as the increased final weight of broilers [25].
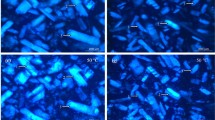
Computer image analysis revealed statistically significant differences in the histological image of two healthy pectoral muscles, i.e., the pectoralis major muscle A (Fig. 2a) and pectoralis minor muscle B (Fig. 2b) (Table 1). The pectoralis minor muscle fibres (Fig. 2b) were characterised by a smaller cross-sectional area than the superficial muscle (Fig. 2a). They had a larger number of muscle fibres with a higher percentage share in terms of the aspect studied.
In the breast muscles with symptoms of third-stage DPM myopathy, the changes in the pectoralis major muscles were invisible macroscopically. However, using histopathological analysis, the size heterogeneity of muscle fibre was observed sporadically (Table 1). In the case of wooden breast [26], pathological changes were observed in superficial pectoral myopathy in broiler chicken. Moreover, using macroscopic analysis, in 32-day-old chickens, mildly discoloured, white-striped lesions consistent with muscular fibre were also observed in the superficial pectoral muscles. Histopathologically, hyaline degeneration, floccular degeneration, size heterogeneity, and phagocytosis of muscle fibre were observed sporadically. However, using histopathological analysis, hyaline degeneration, floccular degeneration, size heterogeneity, and phagocytosis of muscle fibre were observed sporadically. Therefore, DPM degenerative symptoms concerned large superficial pectoral muscles like other meat properties abnormalities described above could be called “superficial pectoral myopathy” (SPM). Superficial pectoral myopathy seems to the same as for wooden breast.
The comparison of green-coloured pectoralis minor muscles with DPM (Fig. 2d) and normal-coloured healthy muscles (Fig. 2b) showed that the former were characterised by a smaller average cross-sectional area of muscle fibres (Table 1). These differences were statistically significant. The myopathic muscle was characterised by the smallest percentage of muscle cells in the image field, whereas their number was the largest of all the muscles under investigation. The differences can be seen in the images of the muscle microstructure (Fig. 2). Both the cross-sectional and longitudinal sections of the DPM muscle (Fig. 2d) show large spaces between bundles of muscle fibres, which are surrounded by the perimysium. Similar results were reported in the study by Soglia et al. [27], who assessed the impact of wooden breast abnormality on quality traits of pectoral major muscles. Compared to the normal p. major muscles, the wooden breast fillet was characterised by a smaller number and area of muscle fibres and showed a rounded profile. The study conducted by Sihvo et al. [25] described the macroscopic and histologic lesions of myopathy affecting the pectoralis major muscle of broilers. The cross section of muscles with myopathy revealed cells without the characteristic polygonal shape, whereas individual cells changed into disc-shaped derivatives. Dinev and Kanakov [28] investigated the prevalence of DPM in young broiler chickens according to their live weight during slaughtering inspection. They showed that the muscle tissue was almost completely replaced by fibrous and/or adipose tissue as a result of degeneration.
DSC analysis
Figure 3a, b shows the DSC curves of tissue denaturation in m. pectoralis major and m. pectoralis minor chicken. The thermal stability of muscle tissue proteins and the degree of their nativity were tested by means of differential scanning calorimetry. Figure 3a shows the curves observed in the normal pectoralis minor muscle and the muscle with DPM. There are five peaks, i.e., T1, T2, T3, T4, and T5. There are also five peaks in the pectoralis major muscle (Fig. 3b), but they are not so clearly separated. Apart from this, it is noticeable that the myopathic muscles were much less intensely denatured than normal ones. The peaks were smaller, which may have been caused by the partial denaturation of proteins as a consequence of muscle necrosis. Table 2 shows the parameters of temperatures, enthalpies, and peak values observed in DSC curves. The temperatures of three most noticeable peaks were found: T1—corresponding to myosin denaturation, T2—collagen-specific peak, and T3—actin-specific peak [29, 30]. In addition, the temperature range of transformation ΔT was calculated as the difference between the transformation-end temperature and the transformation-onset temperature. The statistical analysis showed statistically significant differences only in the myopathic pectoralis minor muscle. In comparison with the normal muscle, temperature T1 of the minor muscle with DPM was lower by 5.9 °C, temperature T2 by 6.7 °C, and T3 –by 7.6 °C. There were no significant differences in the transformation temperature range ΔT, but it was 1.5 °C lower in the pectoralis minor muscle with DPM. The degree of protein nativity was assessed by means of the denaturing transformation enthalpy parameter ΔH, which was calculated according to the area under the DSC curve. The enthalpy values noted in the pectoralis major and minor muscles with DPM were significantly lower than the enthalpy values noted in the healthy muscles. The lowest value, i.e., 1.81 J/g was noted in the pectoralis minor muscle with DPM. It amounted to 38% of the value noted in the healthy minor muscle. The enthalpy value noted in the major muscle with DPM was 3.55 J/g, which amounted to 84% of the value noted in the normal muscle. Table 1 also shows the peak value parameter. As can be seen in thermograms (Fig. 3a, b), there were considerable differences in the transformation intensity between healthy muscles and those with DPM. The transformation intensity parameter was calculated for the values of the first (myosin ΔY1) and last peak (actin ΔY5). For both normal chicken muscles, pectoralis major and pectoralis minor, the myosin peak transformation intensity parameter ΔY1 did not show significant differences, but there were significant differences in the DPM muscles. The lowest transformation intensity was noted in the minor muscle with DPM as it amounted to 29% of the value noted in the normal minor muscle. The ΔY1 value noted in the major muscle with DPM amounted to 79% of the value noted in the normal major muscle. Actin denaturation was characterised by less drastic variations than myosin. The ΔY5 value noted in the minor muscle with DPM was significantly lower, as it amounted to 57% of the value noted in the normal muscle. The ΔY5 value noted in the major muscle with DPM amounted to 87% of the value noted in the normal major muscle.
Measurement of the dynamics of water molecules using LF NMR
The values of spin–lattice relaxation times T1 correspond to the ratio between free water and the water firmly held in the system being analysed [31]. The longer the relaxation time T1, the higher the content of free water in the system. The systems being studied were always characterised by one relaxation time. This means that there was a rapid proton exchange between free water and firmly held water molecules. Spin–spin relaxation times (T2) describe the dynamics of molecules with protons. There are two fractions of protons with different spin–spin relaxation times observed in muscles tested by means of low-field nuclear magnetic resonance [23]. The short component of the spin–spin relaxation time describes the mobility of molecules in the fixed water fraction. The values of the long component of the spin–spin relaxation time indicate the molecular dynamics of free water. The results of our study were identical with those observed by the other researchers analysing the molecular properties of water in muscle systems. Changes in the muscle microstructure directly affect water distribution among three water populations defined by nuclear magnetic resonance (NMR) T2 relaxation studies. These are: T2B (water closely firmly held with macromolecules/proteins), T21 (water trapped into myofibrillar matrix), and T22 (extramyofibrillar water), with each water compartment exhibiting its typical relaxation time [19]. The results presented in this study do not allow for the fraction of water protons which are directly embedded in the biopolymer structure. The very short relaxation time of this water fraction could not be observed with the PST15 spectrometer used in the research because of the long dead time of the radio pulse. Nevertheless, NMR analysis enabled assessment of the water fixation by proteins by calculating the spin–lattice relaxation times (T1).
Table 3 shows the values of relaxation times in the muscles under study. All the values of the short component of the spin–spin relaxation times (T21) were very similar to each other. This means that there was minimal dependence between the molecular dynamics of firmly held water and the muscle type or progression of pathological lesions. Comparison of the spin–lattice relaxation times (T1) showed that the values of this parameter in the normal pectoralis major muscles (A) were significantly greater than the values observed in the myopathic muscles (C). The comparison of pectoralis minor muscles, i.e., samples B and D, revealed an inverse dependence. Simultaneously, there was lesser molecular mobility of free water in the healthy pectoralis major muscle (T1 = 419 ms) than in the healthy pectoralis minor muscle (T1 = 436 ms). It was manifested by a higher value of the long component of the spin–spin relaxation time (T22 = 189 ms).
The interrelations between the average values of relaxation times T1 and T22 calculated for healthy and DPM muscles were also analysed. The value of the long component of relaxation time T22 was reduced in pathologically lesioned major and minor muscles. This resulted in limited migration of free water molecules, which may have been caused by structural changes in proteins. It is likely that these changes were the consequence of histological changes observed macroscopically. Pathological lesions in the samples of pectoralis major muscles (A and C) were manifested by a reduced spin–lattice relaxation time (T1). The analysis of minor muscles (B and D) showed that pathological lesions extended relaxation time T1 significantly, meaning that the pathological system contained more free water than the system without myopathy. Soglia et al. [32] suggested that the wooden breast condition resulted in a remarkable decrease in the intramyofibrillar fraction and a concomitant increase in the extramyofibrillar water fraction.
The research findings showed that the pectoral muscles differed in the molecular organisation of water. Differences in the values of relaxation parameters might suggest the following mechanism of changes in the dynamic state of water caused by pathological lesions. Water was removed into the intramyofibrillar space in the deep muscle with DPM. It may have been caused by conformation changes in proteins. At the same time, if similar effects were observed in water molecules in the superficial muscle, free water was probably evacuated outside the macroscopic system in the form of a drip. Therefore, the amount of free water was reduced, which resulted in a lower T1 value. Conformation changes in muscle proteins could also be proved by the aforementioned limited molecular dynamics of free water. It is most likely that these changes were caused by the process of coagulation and/or denaturation occurring in myofibrillar proteins (especially myosin) of myopathic muscles.
Conclusion
It seems significant that all the research findings point to the occurrence of unfavourable changes both in pectoralis major and minor muscles with DPM. Computer image analysis shows the histological traits of muscle fibres with a wide range of degenerative lesions in the structure, especially in the fibres of pectoralis minor muscles with DPM. At the same time, the cross section and longitudinal section of pectoralis minor muscle with DPM demonstrate numerous large spaces between bundles of muscle fibres. The DSC analysis clearly indicates that myopathy caused unfavourable changes in the native protein structure of muscle. Thermal transformations in pectoral muscles with DPM are significantly less intense than in normal muscles. The significance of transformations occurring in pectoral muscles with myopathy is also proved by the results of LF NMR analysis. A comparison of spin–lattice relaxation times (T1) shows that there were significantly higher values of this parameter in healthy pectoralis major muscles than in the muscles with myopathy. Unfavourable changes in the histological structure of muscle fibres and the native state of proteins, especially myosin, may have caused the redistribution of water in the systems being studied.
Despite the lack of visual changes in surface muscle, undesirable changes of quality caused by DPM defect were observed. It is likely that changes in the structure of chicken DPM muscles have an influence on their functional properties and possible ways of technological usage. Instrumental methods used in this study can be used to identify the changes described and show promise for developing tools to improve scientific relevance of chicken breast muscles affected by DPM abnormality.
References
OECD-FAO Agricultural (2016) Outlook 2016–2025. OECD Publishing, Paris
National Chicken Council. U.S (2015) Broiler performance statistics. http://www.nationalchickencouncil.org/about-the-industry/statistics/u-s-broiler performance/. Accessed May 2015
Mitchell MA, Sandercock DA (2004) Spontaneous and stress induced myopathies in modern meat birds: a cause for quality and welfare concerns. Pages 100–107. In: Proceedings—Australian Poultry Science Symposium. February 9–11, Sydney, Australia
Kijowski J, Kupinska E (2013) The evaluation of selected quality parameters of broiler chicken muscules with deep pectoral myopathy (DPM) symptoms. Arch Geflugelkd 77:102–108
Bailey RB, Watson KA, Bilgili SF, Avendando S (2015) The genetic basis of pectoralis major myopathies in modern broiler chicken lines. Poult Sci 94:2870–2879
Bianchi M, Petracci M, Franchini A, Cavani C (2006) The occurrence of deep pectoral myopathy in roaster chickens. Poult Sci 85:1843–1846
Kijowski J, Konstańczak M (2009) Deep pectoral myopathy in broiler chickens. Bull Vet Ins Pulawy 53:487–491
Bilgili SF, Hess JB (2008) Green muscle disease—reducing the incidence in broiler flocks. Aviagen Brief 3:2–5
Kijowski J, Kupińska E, Stangierski J, Tomaszewska-Gras J, Szablewski T (2014) Paradigm of deep pectoral myopathy in broiler chickens. World’s Poult Sci J 1:125–138
Petracci M, Cavani C (2012) Muscle growth and poultry quality issues. Nutrients 4:1–12
Bilgili SF (2016) Breast muscle abnormalities in broiler chickens. American Association of Avian Pathologists, Inc., Veterinarians, Florida, USA, pp 1–5
Yalcin S, Şahin K, Tuzcu M, Bilgen G, Őzkan S, Izzetoğlu GT, Işik R (2018) Muscle structure and gene expression in pectoralis major muscle in response to deep pectoral myopathy induction in fast- and slow-growing commercial broilers. Brit Poultry Sci. https://doi.org/10.1080/00071668.2018.1430351
Smith DP (2013) New tools for the measurement of poultry quality. World’s Poult Sci J 69:1–6 (Supl)
Xiong YL, Brekke CJ, Leung HK (1987) Thermal denaturation of muscle proteins from different species and muscle types as studied by differential scanning calorimetry. Can Inst Food Sci 20:357–362
Voutila L, Ruusunen M, Jouppila K, Puolanne E (2009) Thermal properties of connective tissue in breast and leg muscles of chickens and turkeys. J Sci Food Agr 89(5):890–896
Tomaszewska-Gras J, Konieczny P (2012) Effect of marination on the thermodynamic properties of chicken muscle proteins studied by DSC. Czech J Food Sci 30:302–308
Skipnes D, Van der Plancken I, Van Loey A, Hendrickx ME (2008) Kinetics of heat denaturation of proteins from farmed Atlantic cod (Gadus morhua). J Food Eng 85:51–58
Konieczny P, Tomaszewska-Gras J, Andrzejewski W, Mikołajczak B, Urbanska M, Mazurkiewicz J, Stangierski J (2016) DSC and electrophoretic studies on protein denaturation of Anodonta woodiana (Lea, 1834). J Therm Anal Calorim 126:69–75
Tasoniero G, Bertram HC, Young JF, Zotte AD, Puolanne E (2017) Relationship between hardness and myowater properties in Wooden Breast affected chicken meat: a nuclear magnetic resonance study. LWT Food Sci Technol 86:20–24
Kijowski J, Kupińska E (2012) Induction of DPM changes in broiler chickens and characteristics of myopathy symptoms. Bull Vet Inst Pulawy 56:217–223
Brosio E, Gianferri R (2009) Low-resolution NMR—an analytical tool in foods characterization and traceability. In: Brosio E (ed) Basic NMR in foods characterization. Research Signpost Kerala, India, pp 9–37
Węglarz WP, Harańczyk H (2000) Two-dimensional analysis of the nuclear relaxation function in the time domain: the CracSpin program. J Physics D Appl Phys 33:1909–1920
Baranowska HM (2011) Water molecular properties in forcemeats and finely ground sausages containing plant fat. Food Biophys 6:133–137
Berri C, Le Bihan-Duval E, Debut M, Sante-Lhoutellier V, Baeza E, Gigaud V, Jego Y, Duclos MJ (2007) Consequence of muscle hypertrophy on characteristics of Pectoralis major muscle and breast meat quality of broiler chickens. J Anim Sci 85:2005–2011
Sihvo HK, Immonen K, Puolanne E (2014) Myodegeneration with fibrosis and regeneration in the Pectoralis major muscle of broilers. Vet Pathol 51:619–623
Otsu M, Kuramochi K, Sasaki J, Ochiai K, Goryo M (2017) Pathological study of superficial pectoral myopathy in broiler chickens. Farm Anim Med Anim Health 6:357–362
Soglia F, Mudalal S, Babini E, Di Nunzio M, Mazzoni M, Sirri F, Cavani C, Petracci M (2016) Histology, composition, and quality traits of chicken Pectoralis major muscle affected by wooden breast abnormality. Poult Sci 95:651–659
Dinev I, Kanakov D (2011) Deep pectoral myopathy: prevalence in 7 weeks old broiler chickens in Bulgaria. Rev Med Vet 162:279–283
Kijowski J, Mast MG (1988) Thermal properties of proteins in chicken broiler tissues. J Food Sci 53:363–366
Stangierski J, Zabielski J, Grześ B (2013) Modification of functional quality of raw myofibril preparation obtained from water-washed mechanically recovered chicken meat. Eur Food Res Technol 236:449–458
Stangierski J, Baranowska HM (2015) The influence of heating and cooling process on the water binding in transglutaminase modified chicken protein preparation. Food Bioprocess Technol 8:2359–2367
Soglia F, Laghi L, Canonico L, Cavani C, Petracci M (2016) Functional property issues in broiler breast meat related to emerging muscle abnormalities. Food Res Inter 313:1071–1076
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that have no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Stangierski, J., Tomaszewska-Gras, J., Baranowska, H.M. et al. The effect of deep pectoral myopathy on the properties of broiler chicken muscles characterised by selected instrumental techniques. Eur Food Res Technol 245, 459–467 (2019). https://doi.org/10.1007/s00217-018-3177-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-018-3177-2