Abstract
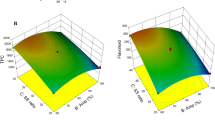
Grape skins are one of the most important leftovers of grape juice production, and are also a good source of bioactive compounds, especially phenolic antioxidants and fiber, because they are not stressed as the winemaking process occurs. Their extracts may be used as functional components of enriched foods and beverage, both to color the products and to supplement with bio-functional metabolites. Therefore, in this work, ultrasound assisted extraction (UAE) and microwave assisted extraction (MAE) were optimized and compared using response surface methodology (RSM) and desirability function (D) statistical tools, at selected temperature and solvent type (close to 50 °C and water/ethanol/phosphoric acid 70:30:1) but varying contact time (t) and sample-to-solvent ratio (S/L), to find the best conditions for the extraction of the main polyphenols present in table grape skin (Apulia Rose cv.) residues from juice processing. The mathematical models built in this investigation showed that the highest significant factor (P < 0.001) was t, influencing the extraction of all compounds irrespective of the technique used, with the optimal results obtained at intermediate levels (10.5 and 21 min for MAE and UAE, respectively). On the contrary, the only S/L factor was not always significant, even though higher amount of polyphenols were generally recovered at low solid/liquid ratio (0.05 and 0.07 g/mL for MAE and UAE, respectively). Finally, UAE extracts exhibited higher content of anthocyanins, procyanidins, flavonols, and stilbenes than MAE, with values ranging from 1.5 to 69.6 mg/100 g of fresh weight.


Similar content being viewed by others
Abbreviations
- HPLC–DAD–MS/MS:
-
High performance liquid chromatography–diode array detector–tandem mass spectrometry
- ESI:
-
Electrospray ionization
- EIC:
-
Extracted ions chromatogram
- CID:
-
Collision induced dissociation
- UV–Vis:
-
Ultraviolet–visible
- [M-H]− :
-
Deprotonated molecule
- [M]+ :
-
Molecular ion
- t :
-
Extraction time
- S/L :
-
Sample-to-solvent ratio
- MAE:
-
Microwave assisted extraction
- UAE:
-
Ultrasound assisted extraction
- RSM:
-
Response surface methodology
- D :
-
Desirability function
References
Medouni-Adrar S, Boulekbache-Makhlouf L, Cadot Y, Medouni-Haroune L, Dahmoune F, Makhoukhe A, Madani K (2015) Ind Crops Prod 77:123–132
O.I.V. (2015). http://www.oiv.int/en/databases-and-statistics/statistics. Accessed 20 Oct 2017
Galanakis CM (2012) Trends Food Sci Technol 26:68–87
Kammerer DR, Kammerer J, Valet R, Carle R (2014) Food Res Int 65:2–12
Karabegović IT, Stojičević SS, Veličković DT, Todorović ZB, Nikolić N, Lazić ML (2014) Ind Crops Prod 54:142–148
Crupi P, Coletta A, Milella RA, Perniola R, Gasparro M, Genghi R, Antonacci D (2012) J Food Sci 77:C174–C181
Crupi P, Pichierri A, Basile T, Antonacci D (2013) Food Chem 141:802–808
Crupi P, Bergamini C, Perniola R, Dipalmo T, Clodoveo ML, Antonacci D (2015) Eur Food Res Technol 241:487–496
Carrieri C, Milella RA, Incampo F, Crupi P, Antonacci D, Semeraro N, Colucci M (2013) Food Chem 140:647–653
Tagliazucchi D, Verzelloni E, Bertolini D, Conte A (2010) Food Chem 120:599–606
Dahmoune F, Boulekbache L, Moussi K, Aoun O, Spigno G, Madani K (2013) Ind Crops Prod 50:77–87
Pinelo M, Rubilar M, Jerez M, Sineiro J, José Nunez M (2005) J Agric Food Chem 53:2111–2117
Wong-Paz JE, Contreras-Esquivel JC, Muniz-Marquez D, Belmares R, Rodriguez R, Flores P, Aguilar CN (2014) Am J Agric Biol Sci 9:299–310
Mané C, Souquet JM, Ollé D, Verriés C, Véran F, Mazerolles G, Cheynier V, Fulcrand H (2007) J Agric Food Chem 55:7224–7233
Wang L, Weller CL (2006) Trends Food Sci Technol 17:300–312
Drosou C, Kyriakopoulou K, Bimpilas A, Tsimogiannis D, Krokida M (2015) Ind Crops Prod 75:141–149
Santos HM, Capelo JL (2007) Talanta 73:795–802
Carrera C, Ruiz-Rodríguez A, Palma M, Barroso CG (2012) Anal Chim Acta 732:100–104
Novak I, Janeiro P, Seruga M, Oliveira-Brett AM (2008) Anal Chim Acta 630:107–115
Pérez-Serradilla JA, Japón-Luján R, Luque de Castro MD (2007) Anal Chim Acta 602:82–88
Dahmoune F, Spigno G, Moussi K, Remini H, Cherbal A, Madani K (2014) Ind Crops Prod 61:31–40
Derringer G, Suich R (1980) J Qual Tech 12:214–219
Al Bittar S, Périno-Issartier S, Dangles O, Chemat F (2013) Food Chem 141:3268–3272
Aspé E, Fernández K (2011) Ind Crops Prod 34:838–844
Spigno G, De Faveri DM (2009) J Food Eng 93:210–217
Spigno G, Tramelli L, De Faveri DM (2007) J Food Eng 81:200–208
Trošt K, Klančnik A, Vodopivec BM, Lemut MS, Novšak KJ, Možina SS (2016) J Sci Food Agric 96:4809–4820
Nuutila AM, Kammiovirta K, Oksman-Caldentey KM (2002) Food Chem 76:519–525
Nicoué EE, Savard S, Belkacemi K (2007) J Agric Food Chem 55:5626–5635
Wang J, Sun B, Cao Y, Tian Y, Li X (2008) Food Chem 106:804–810
Corrales M, Toepfl S, Butz P, Knorr D, Tausher B (2008) Inn Food Sci Emerg Technol 9:85–91
Fabre N, Rustan I, de Hoffmann E, Quentin-Leclercq J (2001) J Am Soc Mass Spectrom12:707–715
Cacace JE, Mazza G (2003) J Food Sci 68:240–248
Liazid A, Guerrero RF, Cantos E, Palma M, Barroso CG (2011) Food Chem 124:1238–1243
Ghafoor K, Choi YH, Jeon JY, Jo IH (2009) J Agric Food Chem 57:4988–4994
Butković V, Klasinc L, Bors W (2004) J Agric Food Chem 52:2816–2820
Liazid A, Palma M, Brigui J, Barroso CG (2007) J Chromatogr A 1140:29–34
Ince AE, Sahin S, Sumnu G (2014) J Food Sci Technol 51:2776–2782
Acknowledgements
This study was supported by grant from the Italian Ministry of University and Research-MIUR (PON02_00186_2937475, Pro.Ali.Fun project).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Compliance with ethics requirements
This article does not contain any studies with human or animal subjects.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Crupi, P., Dipalmo, T., Clodoveo, M.L. et al. Seedless table grape residues as a source of polyphenols: comparison and optimization of non-conventional extraction techniques. Eur Food Res Technol 244, 1091–1100 (2018). https://doi.org/10.1007/s00217-017-3030-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-017-3030-z