Abstract
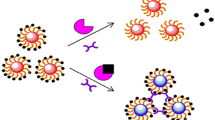
Herein, the self-assembly of 1-dodecanethiol-capped Cu nanoclusters (DT-Cu NCs) is obtained by annealing of dibenzyl ether solution of nanoclusters. These aggregates are composed of small clusters and emit a high level of aggregation-induced emission (AIE) in water. Based on the quenching effect of 4-nitrophenol (4-NP) on DT-Cu NCs, a fluorescence strategy is developed to monitor α-glucosidase (α-Glu) activity and screen its inhibitors from Chinese herbal medicines. 4-Nitrophenyl-α-D-glucopyranoside (NGP) is selected as the substrate, which is further hydrolyzed to yield 4-NP through the catalysis of α-Glu. The quenching efficiency is positively correlated to the concentration of α-Glu. Furthermore, the inhibitory effects of the extracts from four Chinese herbal medicines (i.e., the rind of Punica granatum L., Momordica grosvenorii Swingle., Crataegus pinnatifida Bge., and Lycium barbarum L.) on the α-Glu activity have been studied. The IC50 values of extracts from the rind of Punica granatum L. and Momordica grosvenorii Swingle are 0.23 and 0.37 g/L, respectively, so they show obvious inhibitory effects on α-Glu. The extracts of Crataegus pinnatifida Bge. and Lycium barbarum L. exhibit relatively weak inhibitory effects. Hence, the proposed strategy can be applicable for screening α-Glu inhibitors from Chinese herbal medicines. Last but not the least, by immobilizing DT-Cu NCs into agarose hydrogels in polyethylene tubes, a visual device is fabricated to screen α-Glu inhibitors with high throughput and sensitivity.
Graphical abstract










Similar content being viewed by others
References
Zheng Y, Ley SH, Hu FB. Global aetiology and epidemiology of type 2 diabetes mellitus and its complications. Nat Rev Endocrinol. 2018;14(2):88–98. https://doi.org/10.1038/nrendo.2017.151.
Kurihara H, Ando J, Hatano M, Kawabata J. Sulfoquinovosyldiacylglycerol as an α-glucosidase inhibitor. Bioorg Med Chem Lett. 1995;5(12):1241–4. https://doi.org/10.1016/0960-894X(95)00196-Z.
Van De Laar FA, Lucassen PL, Akkermans RP, Van De Lisdonk EH, Rutten GE, Van Weel C. α-Glucosidase inhibitors for patients with type 2 diabetes: results from a Cochrane systematic review and meta-analysis. Diabetes Care. 2005;28(1):154–63. https://doi.org/10.2337/diacare.28.1.154.
Kong W, Wu D, Xia L, Chen X, Li G, Qiu N, et al. Carbon dots for fluorescent detection of alpha-glucosidase activity using enzyme activated inner filter effect and its application to anti-diabetic drug discovery. Anal Chim Acta. 2017;973:91–9. https://doi.org/10.1016/j.aca.2017.03.050.
Tang C, Qian Z, Qian Y, Huang Y, Zhao M, Ao H, et al. A fluorometric and real-time assay for α-glucosidase activity through supramolecular self-assembly and its application for inhibitor screening. Sensors Actuators B Chem. 2017;245:282–9. https://doi.org/10.1016/j.snb.2017.01.150.
Mondal A, Jana NR. Fluorescent detection of cholesterol using beta-cyclodextrin functionalized graphene. Chem Commun. 2012;48(58):7316–8. https://doi.org/10.1039/c2cc33410k.
Hu QD, Tang GP, Chu PK. Cyclodextrin-based host-guest supramolecular nanoparticles for delivery: from design to applications. Acc Chem Res. 2014;47(7):2017–25. https://doi.org/10.1021/ar500055s.
Munir S, Park SY. The development of a cholesterol biosensor using a liquid crystal/aqueous interface in a SDS-included beta-cyclodextrin aqueous solution. Anal Chim Acta. 2015;893:101–7. https://doi.org/10.1016/j.aca.2015.08.051.
Guo Y, Cao F, Lei X, Mang L, Cheng S, Song J. Fluorescent copper nanoparticles: recent advances in synthesis and applications for sensing metal ions. Nanoscale. 2016;8(9):4852–63. https://doi.org/10.1039/c6nr00145a.
Jia X, Li J, Wang E. Cu nanoclusters with aggregation induced emission enhancement. Small. 2013;9(22):3873–9. https://doi.org/10.1002/smll.201300896.
Wang C, Cheng H, Huang Y, Xu Z, Lin H, Zhang C. Facile sonochemical synthesis of pH-responsive copper nanoclusters for selective and sensitive detection of Pb2+ in living cells. Analyst. 2015;140(16):5634–9. https://doi.org/10.1039/C5AN00741K.
Jia X, Yang X, Li J, Li D, Wang E. Stable Cu nanoclusters: from an aggregation-induced emission mechanism to biosensing and catalytic applications. Chem Commun. 2014;50(2):237–9. https://doi.org/10.1039/c3cc47771a.
Zhao M, Feng H, Han J, Ao H, Qian Z. Multi-stimuli responsive copper nanoclusters with bright red luminescence for quantifying acid phosphatase activity via redox-controlled luminescence switch. Anal Chim Acta. 2017;984:202–10. https://doi.org/10.1016/j.aca.2017.06.046.
Li Z, Guo S, Lu C. A highly selective fluorescent probe for sulfide ions based on aggregation of Cu nanocluster induced emission enhancement. Analyst. 2015;140(8):2719–25. https://doi.org/10.1039/C5AN00017C.
Lin YJ, Chen PC, Yuan Z, Ma JY, Chang HT. The isomeric effect of mercaptobenzoic acids on the preparation and fluorescence properties of copper nanoclusters. Chem Commun. 2015;51(60):11983–6. https://doi.org/10.1039/c5cc02342d.
Huang Y, Liu W, Feng H, Ye Y, Tang C, Ao H, et al. Luminescent nanoswitch based on organic-phase copper nanoclusters for sensitive detection of trace amount of water in organic solvents. Anal Chem. 2016;88(14):7429–34. https://doi.org/10.1021/acs.analchem.6b02149.
Gao M, Tang BZ. Fluorescent sensors based on aggregation-induced emission: recent advances and perspectives. ACS Sensors. 2017;2(10):1382–99. https://doi.org/10.1021/acssensors.7b00551.
Qu F, Xia W, Xia L, You J, Han W. A ratiometric detection of heparin with high sensitivity based on aggregation-enhanced emission of gold nanoclusters triggered by silicon nanoparticles. Talanta. 2019;193:37–43. https://doi.org/10.1016/j.talanta.2018.09.098.
Wu Z, Liu J, Gao Y, Liu H, Li T, Zou H, et al. Assembly-induced enhancement of Cu nanoclusters luminescence with mechanochromic property. J Am Chem Soc. 2015;137(40):12906–13. https://doi.org/10.1021/jacs.5b06550.
Parker AJ, Brody D. 772. Solvation of ions. Part IV. The electronic absorption spectra of some group VI anions and their conjugate acids in protic and dipolar aprotic solvents. J Chem Soc. 1963:4061–8. https://doi.org/10.1039/JR9630004061.
Ai L, Jiang W, Liu Z, Liu J, Gao Y, Zou H, et al. Engineering a red emission of copper nanocluster self-assembly architectures by employing aromatic thiols as capping ligands. Nanoscale. 2017;9(34):12618–27. https://doi.org/10.1039/C7NR03985A.
Min J, Wang F, Cai Y, Liang S, Zhang Z, Jiang X. Azeotropic distillation assisted fabrication of silver nanocages and their catalytic property for reduction of 4-nitrophenol. Chem Commun. 2014;51(4):761–4. https://doi.org/10.1039/C4CC07629J.
Zhao M, Qian Z, Zhong M, Chen Z, Ao H, Feng H. Fabrication of stable and luminescent copper nanocluster-based AIE particles and their application in β-galactosidase activity assay. ACS Appl Mater Interfaces. 2017;9(38):32887–95. https://doi.org/10.1021/acsami.7b09659.
Han L, Liu SG, Liang JY, Ju YJ, Li NB, Luo HQ. pH-mediated reversible fluorescence nanoswitch based on inner filter effect induced fluorescence quenching for selective and visual detection of 4-nitrophenol. J Hazard Mater. 2019;362:45–52. https://doi.org/10.1016/j.jhazmat.2018.09.025.
Zhang J, Zhou R, Tang D, Hou X, Wu P. Optically-active nanocrystals for inner filter effect-based fluorescence sensing: achieving better spectral overlap. Trac-Trend Anal Chem. 2019;110:183–90. https://doi.org/10.1016/j.trac.2018.11.002.
Qu F, Sun C, Lv X, You J. A terbium-based metal-organic framework@gold nanoparticle system as a fluorometric probe for aptamer based determination of adenosine triphosphate. Microchim Acta. 2018;185(8):359. https://doi.org/10.1007/s00604-018-2888-1.
Guo X, Deng L, Wang J. Oligonucleotide-stabilized silver nanoclusters as fluorescent probes for sensitive detection of hydroquinone. RSC Adv. 2013;3(2):401–7. https://doi.org/10.1039/C2RA21615A.
Zhang X, Wu FG, Liu P, Gu N, Chen Z. Enhanced fluorescence of gold nanoclusters composed of HAuCl4 and histidine by glutathione: glutathione detection and selective cancer cell imaging. Small. 2014;10(24):5170–7. https://doi.org/10.1002/smll.201401658.
Wang HB, Chen Y, Li Y, Liu YM. A sensitive fluorescence sensor for glutathione detection based on MnO2 nanosheets–copper nanoclusters composites. RSC Adv. 2016;6(83):79526–32. https://doi.org/10.1039/C6RA17850B.
Zhang J, Liu Y, Wang X, Chen Y, Li G. Electrochemical assay of alpha-glucosidase activity and the inhibitor screening in cell medium. Biosens Bioelectron. 2015;74:666–72. https://doi.org/10.1016/j.bios.2015.07.023.
Li J, He G, Wang B, Shi L, Gao T, Li G. Fabrication of reusable electrochemical biosensor and its application for the assay of alpha-glucosidase activity. Anal Chim Acta. 2018;1026:140–6. https://doi.org/10.1016/j.aca.2018.04.015.
Xi M, Hai C, Tang H, Chen M, Fang K, Liang X. Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytother Res. 2008;22(2):228–37. https://doi.org/10.1002/ptr.2297.
Tong XL, Dong L, Chen L, Zhen Z. Treatment of diabetes using traditional Chinese medicine: past, present and future. Am J Chin Med. 2012;40(5):877–86. https://doi.org/10.1142/S0192415X12500656.
Kim KY, Nam KA, Kurihara H, Kim SM. Potent alpha-glucosidase inhibitors purified from the red alga Grateloupia elliptica. Phytochemistry. 2008;69(16):2820–5. https://doi.org/10.1016/j.phytochem.2008.09.007.
Funding
This work was supported by the National Natural Science Foundation of China (No. 21775088, 21705095), the Natural Science Foundation of Shandong Province, China (ZR2019QB010), and the Scientific Research Foundation of Qufu Normal University (BSQD20130117).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The human serum sample experiments were performed in accordance with the guidelines from the Ethical Committee, Qufu Normal University. All serum samples were obtained from healthy volunteers with their informed consent. All studies were approved by the Ethical Committee of Qufu Normal University.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
ESM 1
(PDF 3801 kb)
Rights and permissions
About this article
Cite this article
Li, C., Zi, Y., Xu, D. et al. A fluorescence strategy for monitoring α-glucosidase activity and screening its inhibitors from Chinese herbal medicines based on Cu nanoclusters with aggregation-induced emission. Anal Bioanal Chem 413, 2553–2563 (2021). https://doi.org/10.1007/s00216-021-03214-w
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-021-03214-w