Abstract
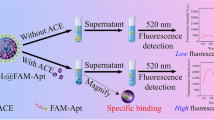
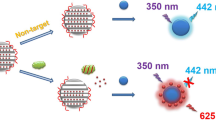
Zearalenone (ZEN) is a type of estrogenic mycotoxin commonly occurring in cereals. The aim of this study was to design a simple, rapid, inexpensive and ultrasensitive fluorescence assay for the determination of ZEN. Here, amino-modified mesoporous silica nanoparticles (MSNs-NH2) were synthesized to be the positive charge-rich reactor. A 6-carboxy-fluorescein-labeled aptamer (aptamer-FAM) was designed as the signal probe, ZEN-capture probe and negative charge reactor. In the absence of ZEN, the negatively charged aptamer-FAM combined with the positively charged MSNs-NH2 in an electrostatic manner. In the presence of ZEN, the fluorescence intensity in the supernatant increased significantly because the aptamer-FAM could bind to ZEN instead of MSNs-NH2. Under the optimal experimental conditions, this assay exhibited excellent specificity, repeatability and a wide linearity range of 0.005–150 ng/mL, with a detection limit of 0.012 ng/mL. Additionally, it showed high recovery (83.3–101.5%) for the spiked samples. There was no statistically significant difference in the ZEN concentrations detected by the proposed assay and HPLC in naturally contaminated samples. Overall, this design provides a new strategy for the rapid, inexpensive and sensitive detection of ZEN, and it could be applied to develop fluorometric assays for different targets by the selection of appropriate aptamers.

Graphical abstract





Similar content being viewed by others
References
Pante GC, Silva MV, Romoli JCZ, Rocha GHO, Bando E, Nerilo SB et al. Occurrence of zearalenone in corn meal commercialized in south region of Brazil and daily intake estimates in the Brazilian population. J. Food Saf. 2019;39(5). https://doi.org/10.1111/jfs.12672.
Meyer H, Skhosana ZD, Motlanthe M, Louw W, Rohwer E. Long term monitoring (2014–2018) of multi-mycotoxins in South African commercial maize and wheat with a locally developed and validated LC-MS/MS Method. Toxins. 2019;11(5). https://doi.org/10.3390/toxins11050271.
Gruber-Dorninger C, Jenkins T, Schatzmayr G. Global mycotoxin occurrence in feed: A ten-year survey. Toxins. 2019;11(7). https://doi.org/10.3390/toxins11070375.
Tashiro F, Ueno Y. Mode of action and metabolism of zearalenone, an estrogenic myco-toxin. Journal of Pharmacobio-Dynamics. 1981;4(4).
Rogowska A, Pomastowski P, Sagandykova G, Buszewski B. Zearalenone and its metabolites: effect on human health, metabolism and neutralisation methods. Toxicon. 2019;162:46–56. https://doi.org/10.1016/j.toxicon.2019.03.004.
Cai G, Pan S, Feng N, Zou H, Gu J, Yuan Y, et al. Zearalenone inhibits T cell chemotaxis by inhibiting cell adhesion and migration related proteins. Ecotox Environ Safe. 2019;175:263–71. https://doi.org/10.1016/j.ecoenv.2019.03.045.
Gromadzka K, Waskiewicz A, Chelkowski J, Golinski P. Zearalenone and its metabolites: occurrence, detection, toxicity and guidelines. World Mycotoxin J. 2008;1(2):209–20. https://doi.org/10.3920/WMJ2008.x015.
Taghdisi SM, Danesh NM, Ramezani M, Emrani AS, Abnous K. Novel colorimetric aptasensor for zearalenone detection based on nontarget-induced aptamer walker, gold nanoparticles, and exonuclease-assisted recycling amplification. ACS Appl Mater Interfaces. 2018;10(15):12504–9. https://doi.org/10.1021/acsami.8b02349.
Wu SJ, Liu LH, Duan N, Li Q, Zhou Y, Wang ZP. Aptamer-based lateral flow test strip for rapid detection of zearalenone in corn samples. J Agric Food Chem. 2018;66(8):1949–54. https://doi.org/10.1021/acs.jafc.7b05326.
Luo LJ, Ma S, Li LB, Liu XH, Zhang JY, Li X, et al. Monitoring zearalenone in corn flour utilizing novel self-enhanced electrochemiluminescence aptasensor based on NGQDs-NH2-Ru@SiO2 luminophore. Food Chem. 2019;292:98–105. https://doi.org/10.1016/j.foodchem.2019.04.050.
Guo ZM, Wang MM, Wu JZ, Tao FF, Chen QS, Wang QY, et al. Quantitative assessment of zearalenone in maize using multivariate algorithms coupled to Raman spectroscopy. Food Chem. 2019;286:282–8. https://doi.org/10.1016/j.foodchem.2019.02.020.
Tan H, Ma L, Guo T, Zhou H, Chen L, Zhang Y, et al. A novel fluorescence aptasensor based on mesoporous silica nanoparticles for selective and sensitive detection of aflatoxin B1. Anal Chim Acta. 2019;1068:87–95. https://doi.org/10.1016/j.aca.2019.04.014.
Ma L, Guo T, Pan S, Zhang Y. A fluorometric aptasensor for patulin based on the use of magnetized graphene oxide and DNase I-assisted target recycling amplification. Microchim Acta. 2018;185(10):487. https://doi.org/10.1007/s00604-018-3023-z.
Goud KY, Hayat A, Satyanarayana M, Kumar VS, Catanante G, Gobi KV, et al. Aptamer-based zearalenone assay based on the use of a fluorescein label and a functional graphene oxide as a quencher. Microchim Acta. 2017;184(11):4401–8. https://doi.org/10.1007/s00604-017-2487-6.
Jiang M, Chen C, He J, Zhang H, Xu Z. Fluorescence assay for three organophosphorus pesticides in agricultural products based on magnetic-assisted fluorescence labeling aptamer probe. Food Chem. 2020;307. https://doi.org/10.1016/j.foodchem.2019.125534.
Chen Z, Tan Y, Xu K, Zhang L, Qiu B, Guo L, et al. Stimulus-response mesoporous silica nanoparticle-based chemiluminescence biosensor for cocaine determination. Biosens Bioelectron. 2016;75:8–14. https://doi.org/10.1016/j.bios.2015.08.006.
Chen Z, Sun M, Luo F, Xu K, Lin Z, Zhang L. Stimulus-response click chemistry based aptamer-functionalized mesoporous silica nanoparticles for fluorescence detection of thrombin. Talanta. 2018;178:563–8. https://doi.org/10.1016/j.talanta.2017.09.043.
Wang Y, Lu M, Zhu J, Tian S. Wrapping DNA-gated mesoporous silica nanoparticles for quantitative monitoring of telomerase activity with glucometer readout. J Mat Chem B. 2014;2(35):5847–53. https://doi.org/10.1039/c4tb00843j.
Dehghani S, Danesh NM, Ramezani M, Alibolandi M, Lavaee P, Nejabat M, et al. A label-free fluorescent aptasensor for detection of kanamycin based on dsDNA-capped mesoporous silica nanoparticles and Rhodamine B. Anal Chim Acta. 2018;1030:142–7. https://doi.org/10.1016/j.aca.2018.05.003.
Ribes A, Aznar E, Bernardos A, Marcos MD, Amoros P, Martinez-Manez R, et al. Fluorogenic sensing of carcinogenic bisphenol a using aptamer-capped mesoporous silica nanoparticles. Chem-Eur J. 2017;23(36):8581–4. https://doi.org/10.1002/chem.201701024.
Kankala RK, Zhang H, Liu CG, Kanubaddi KR, Lee CH, Wang SB et al. Metal species–encapsulated mesoporous silica nanoparticles: current advancements and latest breakthroughs. Adv. Funct. Mater. 2019;29(43). https://doi.org/10.1002/adfm.201902652.
Niazi S, Wang XL, Pasha I, Khan IM, Zhao S, Shoaib M, et al. A novel bioassay based on aptamer-functionalized magnetic nanoparticle for the detection of zearalenone using time resolved-fluorescence NaYF4: Ce/Tb nanoparticles as signal probe. Talanta. 2018;186:97–103. https://doi.org/10.1016/j.talanta.2018.04.013.
Karimi S, Heydari M. Voltammetric mixture analysis of tyrosine and tryptophan using carbon paste electrode modified by newly synthesized mesoporous silica nanoparticles and clustering of variable-partial least square: efficient strategy for template extraction in mesoporous silica nanoparticle synthesis. Sens. Actuator B-Chem. 2018;257:1134–42. https://doi.org/10.1016/j.snb.2017.11.014.
Kenawy IMM, Abou El-Reash YG, Hassanien MM, Alnagar NR, Mortada WI. Use of microwave irradiation for modification of mesoporous silica nanoparticles by thioglycolic acid for removal of cadmium and mercury. Microporous Mesoporous Mat. 2018;258:217–27. https://doi.org/10.1016/j.micromeso.2017.09.021.
Ribes A, Santiago-Felipe S, Bernardos A, Marcos MD, Pardo T, Sancenon F, et al. Two new fluorogenic aptasensors based on capped mesoporous silica nanoparticles to detect ochratoxin a. ChemistryOpen. 2017;6(5):653–9. https://doi.org/10.1002/open.201700106.
Yokoi T, Kubota Y, Tatsumi T. Amino-functionalized mesoporous silica as base catalyst and adsorbent. Appl Catal A-Gen. 2012;421:14–37. https://doi.org/10.1016/j.apcata.2012.02.004.
Zhang FY, Liu B, Liu GZ, Sheng W, Zhang Y, Liu Q, et al. Novel magnetic nanobeads-based fluoroimmunoassays for zearalenone detection in cereals using protein G as the recognition linker. Sens Actuator B-Chem. 2018;270:149–57. https://doi.org/10.1016/j.snb.2018.04.131.
Zhang F, Liu B, Sheng W, Zhang Y, Liu Q, Li S, et al. Fluoroimmunoassays for the detection of zearalenone in maize using CdTe/CdS/ZnS quantum dots. Food Chem. 2018;255:421–8. https://doi.org/10.1016/j.foodchem.2018.02.060.
Zhan SN, Huang XL, Chen R, Li J, Xiong YH. Novel fluorescent ELISA for the sensitive detection of zearalenone based on H2O2-sensitive quantum dots for signal transduction. Talanta. 2016;158:51–6. https://doi.org/10.1016/j.talanta.2016.05.035.
Chen Y, Fu QQ, Xie J, Wang H, Tang Y. Development of a high sensitivity quantum dot-based fluorescent quenching lateral flow assay for the detection of zearalenone. Anal Bioanal Chem. 2019;411(10):2169–75. https://doi.org/10.1007/s00216-019-01652-1.
Zhao F, Shen Q, Wang H, Han X, Yang Z. Development of a rapid magnetic bead-based immunoassay for sensitive detection of zearalenone. Food Control. 2017;79:227–33. https://doi.org/10.1016/j.foodcont.2017.03.051.
Funding
This work was supported by the Fundamental Research Funds for the Central Universities of China (Project NO. XDJK2020B044) and Venture & Innovation Support Program for Chongqing Overseas Returnees (Project No. cx2018032).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 207 kb)
Rights and permissions
About this article
Cite this article
Tan, H., Guo, T., Zhou, H. et al. A simple mesoporous silica nanoparticle-based fluorescence aptasensor for the detection of zearalenone in grain and cereal products. Anal Bioanal Chem 412, 5627–5635 (2020). https://doi.org/10.1007/s00216-020-02778-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02778-3