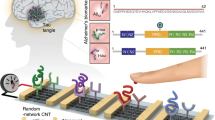
Abstract
Early diagnosis in primary care settings can increase access to therapies and their efficiency as well as reduce health care costs. In this context, we report in this paper the development of a disposable immunoplatform for the rapid and simultaneous determination of two protein biomarkers recently reported to be involved in the pathological process of neurodegenerative disorders (NDD), tau protein (tau), and TAR DNA-binding protein 43 (TDP-43). The methodology involves implementation of a sandwich-type immunoassay on the surface of dual screen-printed carbon electrodes (dSPCEs) electrochemically grafted with p-aminobenzoic acid (p-ABA), which allows the covalent immobilization of a gold nanoparticle-poly(amidoamine) (PAMAM) dendrimer nanocomposite (3D-Au-PAMAM). This scaffold was employed for the immobilization of the capture antibodies (CAbs). Detector antibodies labeled with horseradish peroxidase (HRP) and amperometric detection at − 0.20 V (vs. Ag pseudo-reference electrode) using the H2O2/hydroquinone (HQ) system were used. The developed methodology exhibits high sensitivity and selectivity for determining the target proteins, with detection limits of 2.3 and 12.8 pg mL−1 for tau and TDP-43, respectively. The simultaneous determination of tau and TDP-43 was accomplished in raw plasma samples and brain tissue extracts from healthy individuals and NDD-diagnosed patients. The analysis can be performed in just 1 h using a simple one-step assay protocol and small sample amounts (5 μL plasma and 2.5 μg brain tissue extracts).

Graphical abstract






Similar content being viewed by others
References
Forman MS, Trojanowski JQ, Lee VM. Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med. 2004;10:1055–63.
Gleerup HS, Hasselbalch SG, Simonsen AH. Biomarkers for Alzheimer’s disease in saliva: a systematic review. Dis Markers. 2019;4761054. https://doi.org/10.1155/2019/4761054.
Irwin DJ, Trojanowski JQ, Grossman M. Cerebrospinal fluid biomarkers for differentiation of frontotemporal lobar degeneration from Alzheimer’s disease. Front Aging Neurosci. 2013;5:6. https://doi.org/10.3389/fnagi.2013.00006.
Schneider P, Hampel H, Buerger K. Biological marker candidates of Alzheimer’s disease in blood, plasma, and serum. CNS Neurosci Ther. 2009;15:358–74.
Laske C, Sohrabi HR, Frost SM, Lopez-de-Ipiña K, Garrard P, Buscema M, et al. Innovative diagnostic tools for early detection of Alzheimer’s disease. Alzheimers Dement. 2015;11:561–78.
O’Bryant SE, Xiao G, Barber R, Huebinger R, Wilhelmsen K, Edwards M, et al. A blood-based screening tool for Alzheimer’s disease that spans serum and plasma: findings from TARC and ADNI. PLoS One. 2011;6:e28092. https://doi.org/10.1371/journal.pone.0028092.
O’Bryant SE, Edwards M, Johnson L, Hall J, Villarreal AE, Britton GB, et al. A blood screening test for Alzheimer’s disease. Alzheimers Dement. 2016;3:83–90.
Shi L, Baird AL, Westwood S, Hye A, Dobson R, Thambisetty M, et al. A decade of blood biomarkers for Alzheimer’s disease research: an evolving field, improving study designs, and the challenge of replication. J Alzheimers Dis. 2018;62:1181–98.
Zipser BD, Johanson CE, Gonzalez L, Berzin TM, Tavares R, Hulette CM, et al. Microvascular injury and blood-brain barrier leakage in Alzheimer’s disease. Neurobiol Aging. 2007;28:977–86.
Zetterberg H, Burnham SC. Blood-based molecular biomarkers for Alzheimer’s disease. Mol Brain. 2019;12:26. https://doi.org/10.1186/s13041-019-0448-1.
McAvoy T, Lassman ME, Spellman DS, Ke Z, Howell BJ, Wong O, et al. Quantification of tau in cerebrospinal fluid by immunoaffinity enrichment and tandem mass spectrometry. Clin Chem. 2014;60:e683–9.
Kametani F, Obi T, Shishido T, Akatsu H, Murayama S, Saito Y, et al. Mass spectrometric analysis of accumulated TDP-43 in amyotrophic lateral sclerosis brains. Sci Rep. 2016;6:23281. https://doi.org/10.1038/srep23281.
Meeter LH, Kaat LD, Rohrer JD, van Swieten JC. Imaging and fluid biomarkers in frontotemporal dementia. Nat Rev Neurol. 2017;13:406–19.
Hye A, Riddoch-Contreras J, Baird AL, Ashton NJ, Bazenet C, Leung R, et al. Plasma proteins predict conversion to dementia from prodromal disease. Alzheimers Dement. 2014;10:799–807.
Law WP, Wang WY, Moore PT, Mollee PN, Ng AC. Cardiac amyloid imaging with 18F-florbetaben PET: a pilot study. J Nucl Med. 2016;57:1733–9.
Foulds PG, Davidson Y, Mishra M, Hobson DJ, Humphreys KM, Taylor M, et al. Plasma phosphorylated-TDP-43 protein levels correlate with brain pathology in frontotemporal lobar degeneration. Acta Neuropathol. 2009;118:647–58.
Hosokawa M, Arai T, Yamashita M, Tsuji H, Nonaka T, Masuda-Suzukake M, et al. Differential diagnosis of amyotrophic lateral sclerosis from Guillain–Barre´ syndrome by quantitative determination of TDP-43 in cerebrospinal fluid. Int J Neurosci. 2014;124:344–9.
Junttila A, Kuvaja M, Hartikainen P, Siloaho M, Helisalmi S, Moilanen V, et al. Cerebrospinal fluid TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis patients with and without the C9ORF72 hexanucleotide expansion. Dement Geriatr Cogn Dis Extra. 2016;6:142–9.
Guntert A, Campbell J, Saleem M, O’Brien DP, Thompson AJ, Byers HL, et al. Plasma gelsolin is decreased and correlates with rate of decline in Alzheimer’s disease. J Alzheimers Dis. 2010;21:585–96.
Steinacker P, Hendrich C, Sperfeld AD, Jesse S, von Arnim CA, Lehnert S, et al. TDP-43 in cerebrospinal fluid of patients with frontotemporal lobar degeneration and amyotrophic lateral sclerosis. Arch Neurol. 2008;65:1481–7.
Arai T, Mackenzie IRA, Hasegawa M, Nonoka T, Niizato K, Tsuchiya K, et al. Phosphorylated TDP-43 in Alzheimer’s disease and dementia with Lewy bodies. Acta Neuropathol. 2009;117:125–36.
Shui B, Tao D, Florea A, Cheng J, Zhao Q, Gu Y, et al. Biosensors for Alzheimer's disease biomarker detection: a review. Biochimica. 2018;147:13–24.
Saleem M. Biosensors a promising future in measurements. IOP Conf Ser Mater Sci Eng. 2013;51. https://doi.org/10.1088/1757-899X/51/1/012012.
Scarano S, Lisi S, Ravelet C, Peyrin E, Minunni M. Detecting Alzheimer’s disease biomarkers: from antibodies to new biomimetic receptors and their application to established and emerging bioanalytical platforms – a critical review. Anal Chim Acta. 2016;940:21–37.
Marín S, Merkoçi A. Nanomaterials based electrochemical sensing applications for safety and security. Electroanalysis. 2012;24:459–69.
Luo X, Morrin A, Killard AJ, Smyth MR. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis. 2006;18:319–26.
Mutali SA, Anwar RE, Radji M, Pujiyanto A, Purnamasari P, Joshita D, et al. Synthesis of gold nanoparticles with polyamidoamine (PAMAM) generation 4 dendrimer as stabilizing agent for CT scan contrast agent. Macromol Symp. 2015;353:96–101.
Razzino CA, Serafín V, Gamella M, Pedrero M, Montero-Calle A, Barderas R, et al. An electrochemical immunosensor using gold nanoparticles-PAMAM-nanostructured screen-printed carbon electrodes for tau protein determination in plasma and brain tissues from Alzheimer patients. Biosens Bioelectron. 2020; in press. https://doi.org/10.1016/j.bios.2020.112238.
Umeda Y, Kojima C, Horinaka H, Kono K. PEG-attached PAMAM dendrimers encapsulating gold nanoparticles: growing gold nanoparticles in the dendrimers for improvement of their photothermal properties. Bioconjug Chem. 2010;21:1559–64.
Vasile E, Serafim A, Petre D, Giol D, Dubruel P, Iovu H, et al. Direct synthesis and morphological characterization of gold-dendrimer nanocomposites prepared using PAMAM succinamic acid dendrimers: preliminary study of the calcification potential. Sci World J. 2014. https://doi.org/10.1155/2014/103462.
Luo J, Dong M, Lin F, Liu M, Tang H, Li H, et al. Three-dimensional network polyamidoamine dendrimer-Au nanocomposite for the construction of a mediator-free horseradish peroxidase biosensor. Analyst. 2011;136:4500–6.
Araque E, Arenas CB, Gamella M, Reviejo J, Villalonga R, Pingarrón JM. Graphene–polyamidoamine dendrimer–Pt nanoparticles hybrid nanomaterial for the preparation of mediatorless enzyme biosensor. J Electroanal Chem. 2014;717:96–102.
Borisova B, Sánchez A, Jiménez-Falcao S, Martín M, Salazar P, Parrado C, et al. Reduced graphene oxide-carboxymethylcellulose layered with platinum nanoparticles/PAMAM dendrimer/magnetic nanoparticles hybrids. Application to the preparation of enzyme electrochemical biosensors. Sensors Actuators B Chem. 2016;232:84–90.
Zhang X, Shen J, Ma H, Jiang Y, Huang C, Han E, et al. Optimized dendrimer-encapsulated gold nanoparticles and enhanced carbon nanotube nanoprobes for amplified electrochemical immunoassay of E. coli in dairy product based on enzymatically induced deposition of polyaniline. Biosens Bioelectron. 2016;80:666–73.
Singal S, Srivastava AK, Kotnala, Rajesh RK. Single-frequency impedance analysis of biofunctionalized dendrimer-encapsulated Pt nanoparticles-modified screen-printed electrode for biomolecular detection. J Solid State Electrochem. 2018;22:2649–57.
Liu B, Li M, Zhao Y, Pan M, Gu Y, Sheng W, et al. A sensitive electrochemical immunosensor based on PAMAM dendrimer-encapsulated Au for detection of norfloxacin in animal-derived foods. Sensors. 2018;18:1946. https://doi.org/10.3390/s18061946.
Niu X, Huang L, Zhao J, Yin M, Luo D, Yang Y. An ultrasensitive aptamer biosensor for the detection of codeine based on a Au nanoparticle/polyamidoamine dendrimer-modified screen printed carbon electrode. Anal Methods. 2016;8:1091–5.
An Y, Jiang X, Bi W, Chen H, Jin L, Zhang S, et al. Sensitive electrochemical immunosensor for α-synuclein based on dual signal amplification using PAMAM dendrimer-encapsulated Au and enhanced gold nanoparticle labels. Biosens Bioelectron. 2012;32:224–30.
Pei X, Xu Z, Zhang J, Liu Z, Tian J. Electroactive dendrimer-encapsulated silver nanoparticles for sensing low-abundance proteins with signal amplification. Anal Methods. 2013;5:3235–41.
Hu L, Dong T, Zhao K, Deng A, Li J. Ultrasensitive electrochemiluminescent brombuterol immunoassay by applying a multiple signal amplification strategy based on a PAMAM-gold nanoparticle conjugate as the bioprobe and Ag@Au core shell nanoparticles as a substrate. Microchim Acta. 2017;184:3415–23.
Fang J, Guo Y, Yang Y, Yu W, Tao Y, Dai T, et al. Portable and sensitive detection of DNA based on personal glucose meters and nanogold-functionalized PAMAM dendrimer. Sensors Actuators B Chem. 2018;272:118–26.
Sierks M, Williams S, Venkataraman L. Antibody based reagents that other publications specifically recognize neurodegenerative disease related forms of the protein TPD-43. (2019) Patent No.: US 10, 191, 068 B2: United States of America.
Baird AL, Westwood S, Lovestone S. Blood-based proteomic biomarkers of Alzheimer’s disease pathology. Front Neurol. 2015;6:236. https://doi.org/10.3389/fneur.2015.00236.
Billingsley ML, Kincaid RL. Regulated phosphorylation and dephosphorylation of tau protein: effects on microtubule interaction, intracellular trafficking and neurodegeneration. Biochem J. 1997;323:577–91.
Lee VM-Y, Goedert M, Trojanowski JQ. Neurodegenerative tauopathies. Annu Rev Neurosci. 2001;24:1121–59.
Amador-Ortiz C, Lin WL, Ahmed Z, Personett D, Davies P, Duara R, et al. TDP-43 immunoreactivity in hippocampal sclerosis and Alzheimer’s disease. Ann Neurol. 2007;61:435–45.
Higashi S, Iseki E, Yamamoto R, Minegishi M, Hino H, Fujisawa K, et al. Concurrence of TDP-43, tau and alpha-synuclein pathology in brains of Alzheimer’s disease and dementia with Lewy bodies. Brain Res. 2007;1184:284–94.
Freeman SH, Spires-Jones T, Hyman BT, Growdon JH, Frosch MP. TAR-DNA binding protein 43 in Pick disease. J Neuropathol Exp Neurol. 2008;67:62–7.
Schwab C, Arai T, Hasegawa M, Yu S, McGeer PL. Colocalization of transactivation-responsive DNA-binding protein 43 and huntingtin in inclusions of Huntington disease. J Neuropathol Exp Neurol. 2008;67:1159–65.
Kim YG, Oh SK, Crooks RM. Preparation and characterization of 1-2 nm dendrimer-encapsulated gold nanoparticles having very narrow size distributions. Chem Mater. 2004;16:167–72.
Moreno-Guzmán M, Ojeda I, Villalonga R, González-Cortés A, Yáñez-Sedeño P, Pingarrón JM. Ultrasensitive detection of adrenocorticotropin hormone (ACTH) using disposable phenylboronic-modified electrochemical immunosensors. Biosens Bioelectron. 2012;35:82–6.
Mirra SS, Hart MN, Terry RD. Making the diagnosis of Alzheimer’s disease. A primer for practicing pathologists. Arch Pathol Lab Med. 1993;117:132–44.
Thal DR, Rub U, Orantes M, Braak H. Phases of A beta-deposition in the human brain and its relevance for the development of AD. Neurology. 2002;58:1791–800.
Barderas R, Babel I, Díaz-Uriarte R, Moreno V, Suárez A, Bonilla F, et al. An optimized predictor panel for colorectal cancer diagnosis based on the combination of tumor-associated antigens obtained from protein and phage microarrays. J Proteome. 2012;75:4647–55.
Barderas R, Villar-Vázquez R, Fernández-Aceñero MJ, Babel I, Peláez-García A, Torres S, et al. Sporadic colon cancer murine models demonstrate the value of autoantibody detection for preclinical cancer diagnosis. Sci Rep. 2013;3:2938. https://doi.org/10.1038/srep02938.
Garranzo-Asensio M, San Segundo-Acosta P, Martínez-Useros J, Montero-Calle A, Fernández-Aceñero MJ, Häggmark-Månberg A, et al. Identification of prefrontal cortex protein alterations in Alzheimer’s disease. Oncotarget. 2018;9:10847–67.
Gamella M, Campuzano S, Conzuelo F, Reviejo AJ, Pingarrón JM. Amperometric magnetoimmunosensors for direct determination of D-dimer in human serum. Electroanalysis. 2012;24:2235–43.
Esteves-Villanueva JO, Trzeciakiewicz H, Martic S. A protein-based electrochemical biosensor for detection of tau protein, a neurodegenerative disease biomarker. Analyst. 2014;139:2823–31.
Derkus B, Ozkan M, Emregul KC, Emregul E. Single frequency analysis for clinical immunosensor design. RCS Adv. 2016;6:281–9.
Derkus B, Bozkurt PA, Tulu M, Emregul KC, Yucesan C, Emregul E. Simultaneous quantification of myelin basic protein and tau proteins in cerebrospinal fluid and serum of multiple sclerosis patients using nanoimmunosensor. Biosens Bioelectron. 2017;89:781–8.
Dai Y, Molazemhosseini A, Liu CC. A single-use, in vitro biosensor for the detection of T-tau protein, a biomarker of neuro-degenerative disorders, in PBS and human serum using differential pulse voltammetry (DPV). Biosensors. 2017;7:10. https://doi.org/10.3390/bios7010010.
Wang SX, Acha D, Shah AJ, Hills F, Roitt I, Demosthenous A, et al. Detection of the tau protein in human serum by a sensitive four-electrode electrochemical biosensor. Biosens Bioelectron. 2017;92:82–488.
Shui B, Tao D, Cheng J, Mei Y, Jaffrezic-Renault N, Guo Z. A novel electrochemical aptamer–antibody sandwich assay for the detection of tau-381 in human serum. Analyst. 2018;143:3549–54.
Carlin N, Martic-Milne S. Anti-tau antibodies based electrochemical sensor for detection of tau protein biomarker. J Electrochem Soc. 2018;165:G3018–25.
Tao D, Shui B, Gu Y, Cheng J, Zhang W, Jaffrezic-Renault N, et al. Development of a label-free electrochemical aptasensor for the detection of Tau381 and its preliminary application in AD and non-AD patients’ sera. Biosensors. 2019;9:84. https://doi.org/10.3390/bios9030084.
Ye M, Jiang M, Cheng J, Li X, Liu Z, Zhang W, et al. Single-layer exfoliated reduced graphene oxide-antibody tau sensor for detection in human serum. Sensors Actuators B Chem. 2020;308:127692. https://doi.org/10.1016/j.snb.2020.127692.
Dai Y, Wang C, Chiu LY, Abbasi K, Tolbert BS, Sauvé G, et al. Application of bioconjugation chemistry on biosensor fabrication for detection of TAR-DNA binding protein 43. Biosens Bioelectron. 2018;117:60–7.
Grigorieva DV, Gorudko IV, Sokolov AV, Kosmachevskaya OV, Topunov AF, et al. Measurement of plasma hemoglobin peroxidase activity. Bull Exp Biol Med. 2013;155:118–21.
Zhao RN, Feng Z, Zhao YN, Jia LP, Ma RN, Zhang W, et al. A sensitive electrochemical aptasensor for Mucin1 detection based on catalytic hairpin assembly coupled with PtPdNPs peroxidase-like activity. Talanta. 2019;200:503–10.
Foulds PG, McAuley E, Gibbons L, Davidson Y, Pickering-Brown SM, Neary D, et al. TDP-43 protein in plasma may index TDP-43 brain pathology in Alzheimer’s disease and frontotemporal lobar degeneration. Acta Neuropathol. 2008;116:141–6.
Müller S, Preische O, Göpfert JC, Carcamo Yañez VA, Joos TO, Boecker H, et al. Tau plasma levels in subjective cognitive decline: results from the DELCODE study. Sci Rep. 2017;7:9529. https://doi.org/10.1038/s41598-017-08779-0.
Wilson AC, Dugger BN, Dickson DW, Wang DS. TDP-43 in aging and Alzheimer’s disease – a review. Int J Clin Exp Pathol. 2011;4:147–55.
Steinacker P, Barschke P, Otto M. Biomarkers for diseases with TDP-43 pathology. Mol Cell Neurosci. 2019;97:43–59.
Funding
The financial support of the CTQ2015-64402-C2-1-R (Spanish Ministerio de Economía y Competitividad) and RTI2018-096135-B-I00 (Ministerio de Ciencia, Innovación y Universidades) Research Projects and the TRANSNANOAVANSENS-CM Program from the Comunidad de Madrid (Grant S2018/NMT-4349) are gratefully acknowledged. C.A.R. thanks FAPESP (Grant # 2018/14130-7, Sao Paulo Research Foundation (FAPESP)) for the support granted. R.B. acknowledges the financial support of the PI17CIII/00045 grant from the AES-ISCIII program. A.M-C. was supported by a predoctoral contract of the Fundación Tatiana Perez de Guzman el Bueno and now by an FPU predoctoral contract supported by the Spanish Ministerio de Educación, Cultura y Deporte. E.P. acknowledges a predoctoral contract from the Spanish Ministerio de Economía y Competitividad (BES-2016-076606).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Disclaimer
The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Additional information
Published in the topical collection 2D Nanomaterials for Electroanalysis with guest editor Sabine Szunerits.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 459 kb).
Rights and permissions
About this article
Cite this article
Serafín, V., Razzino, C.A., Gamella, M. et al. Disposable immunoplatforms for the simultaneous determination of biomarkers for neurodegenerative disorders using poly(amidoamine) dendrimer/gold nanoparticle nanocomposite. Anal Bioanal Chem 413, 799–811 (2021). https://doi.org/10.1007/s00216-020-02724-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-020-02724-3