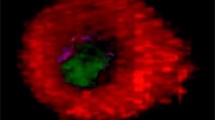
Abstract
Merging optical images of tissue sections with the spatial distributions of molecules seen by imaging mass spectrometry is a powerful approach to better understand the metabolic roles of the mapped molecules. Here, we use histologically friendly desorption electrospray ionization–mass spectrometry (DESI-MS) to map the lipid distribution in tissue sections of ovaries from cows (N = 8), sows (N = 3), and mice (N = 12). Morphologically friendly DESI-MS imaging allows the same sections to be examined for morphological information. Independent of the species, ovarian follicles, corpora lutea, and stroma could be differentiated by principal component analysis, showing that lipid profiles are well conserved among species. As examples of specific findings, arachidonic acid and the phosphatidylinositol PI(38:4), were both found concentrated in the follicles and corpora lutea, structures that promoted ovulation and implantation, respectively. Adrenic acid was spatially located in the corpora lutea, suggesting the importance of this fatty acid in the ovary luteal phase. In summary, lipid information captured by DESI-MS imaging could be related to ovarian structures and data were all conserved among cows, sows, and mice. Further application of DESI-MS imaging to either physiological or pathophysiological models of reproductive conditions will likely expand knowledge of the roles of specific lipids and pathways in ovarian activity and mammalian fertility.

Desorption electrospray ionization–mass spectrometry (DESI-MS) is performed directly from frozen ovarian tissue sections placed onto glass slides. Because the desorption and ionization process of small molecules is so gentle, the tissue architecture is preserved. The sample can then be stained and tissue morphology information can be overlaid with the chemical information obtained by DESI-MS.






Similar content being viewed by others
Data availability
All .raw files and Biomap-compatible DESI-MS imaging files are freely available via MassIVE (MSV00008462), a mass spectrometry data repository.
References
Boots CE, Jungheim ES. Inflammation and human ovarian follicular dynamics. Semin Reprod Med. 2015. https://doi.org/10.1055/s-0035-554928.
Espey LL. Current status of the hypothesis that mammalian ovulation is comparable to an inflammatory reaction. Biol Reprod. 1994;50:233–8.
Smith P, Wilhelm D, Rodgers RJ. Development of mammalian ovary. J Endocrinol. 2014. https://doi.org/10.1530/JOE-14-0062.
Shimizu T, Ishizawa S, Magata F, Kobayashi M, Fricke PM, Miyamoto A. Involvement of lipopolysaccharide in ovarian cystic follicles in dairy cow: expressions of LPS receptors and steroidogenesis-related genes in follicular cells of cystic follicles. Anim Reprod Sci. 2018. https://doi.org/10.1016/j.anireprosci.2018.05.010.
Xu D, He H, Jiang X, Hua R, Chen H, Yang L, et al. SIRT2 plays a novel role on progesterone, estradiol and testosterone synthesis via PPARs/LXRα pathways in bovine ovarian granular cells. J Steroid Biochem Mol Biol. 2019. https://doi.org/10.1016/j.jsbmb.2018.07.005.
Amsterdam A, Keren-Tal I, Aharoni D. Cross-talk beteween cAMP and P53-generated signals in induction of differentiation and apoptosis in steridogenic granulosa cells. Steroids. 1996. https://doi.org/10.1016/0039-128X(96)00031-1.
Takekida S, Deguchi J, Samoto T, Matsuo H, Maruo T. Comparative analysis of the effects of gonadotropin-releasing hormone agonist on the proliferative activity, apoptosis, and steroidogenesis in cultured porcine granulosa cells at varying stages of follicular growth. 2000. https://doi.org/10.1385/ENDO:12:1:61.
Gérard N, Fahiminiya S, Grupen CG, Nadal-Desbarats L. Reproductive physiology and ovarian folliculogenesis examined via 1H-NMR metabolomics signatures: a comparative study of large and small follicles in three mammalian species (Bos taurus, Sus scrofa domesticus and Equus ferus caballus). OMICS. 2015. https://doi.org/10.1089/omi.2014.0097.
Sun L, Chen L, Jiang Y, Zhao Y, Wang F, Zheng X, et al. Metabolomic profiling of ovary in mice treated with FSH using ultra performance liquid chromatography/mass spectrometry. Biosci Rep. 2018. https://doi.org/10.1042/BSR20180965.
Vireque AA, Tata A, Belaz KR, Grázia JG, Santos FN, Arnold DR, et al. MALDI mass spectrometry reveals that cumulus cells modulate the lipid profile of in vitro-matured bovine oocytes. Syst Biol Reprod Med. 2017. https://doi.org/10.1080/19396368.2017.1289279.
Burnum-Johnson KE, Baker ES, Metz TO. Characterizing the lipid and metabolite changes associated with placental function and pregnancy complications using ion mobility spectrometry-mass spectrometry and mass spectrometry imaging. Placenta. 2017. https://doi.org/10.1016/j.placenta.2017.03.016.
Pirro V, Guffey SC, Sepúlveda MS, Mahapatra CT, Ferreira CR, Jarmusch AK, et al. Lipid dynamics in zebrafish embryonic development observed by DESI-MS imaging and nanoelectrospray-MS. Mol BioSyst. 2016. https://doi.org/10.1039/c6mb00168h.
Kolena J. Effect of phospholipase C induced hydrolysis of phospholipids on membrane-bound and water-soluble LH/hCG receptors in porcine corpora lutea. Clin Endocrinol. 1992. https://doi.org/10.1055/s-0029'1211123.
Lee Y, Lee H, Park B, Elahi F, Lee J, Lee ST, et al. Alpha-linolenic acid treatment during oocyte maturation enhances embryonic development by influencing mitogen-activated protein kinase activity and intraoocyte glutathione content in pigs. J Anim Sci. 2016. https://doi.org/10.2527/jas.2016-0384.
Sinderewicz E, Grycmacher K, Boruszewska D, Kowalczyk-Zięba I, Staszkiewicz J, Ślężak T, et al. Expression of factors involved in apoptosis and cell survival is correlated with enzymes synthesizing lysophosphatidic acid and its receptors in granulosa cells originating from different types of bovine ovarian follicles. Reprod Biol Endocrinol. 2017. https://doi.org/10.1186/s12958-017-0298-6.
Takáts Z, Wiseman JM, Gologan B, Cooks RG. Mass spectrometry sampling under ambient conditions with desorption electrospray ionization. Science. 2004. https://doi.org/10.1126/science.1104404.
Wiseman JM, Ifa DR, Venter A, Cooks RG. Ambient molecular imaging by desorption electrospray ionization mass spectrometry. Nat Protoc. 2008. https://doi.org/10.1038/nprot.2008.11.
Dill AL, Ifa DR, Manicke NE, Ouyang Z, Cooks RG. Mass spectrometric imaging of lipids using desorption electrospray ionization. J Chromatogr B Anal Technol Biomed Life Sci. 2009. https://doi.org/10.1016/j.jchromb.2008.12.058.
González-Serrano AF, Pirro V, Ferreira CR, Oliveri P, Eberlin LS, Heinzmann J, et al. Desorption electrospray ionization mass spectrometry reveals lipid metabolism of individual oocytes and embryos. PLoS One. 2013. https://doi.org/10.1371/journal.pone.0074981.
Ferreira CR, Jarmusch AK, Pirro V, Alfaro CM, González-Serrano AF, Niemann H, et al. Ambient ionisation mass spectrometry for lipid profiling and structural analysis of mammalian oocytes, preimplantation embryos and stem cells. Reprod Fertil Dev. 2015. https://doi.org/10.1071/RD14310.
Salama M, Woodruff TK. From bench to bedside: current developments and future possibilities of artificial human ovary to restore fertility. Acta Obstet Gynecol. 2019. https://doi.org/10.1111/aogs.13552.
Eberlin LS, Liu X, Ferreira CR, Santagata S, Agar NYR, Cooks RG. Desorption electrospray ionization then MALDI mass spectrometry imaging of lipid and protein distributions in single tissue sections. Anal Chem. 2011. https://doi.org/10.1021/ac202016x.
Pirro V, Eberlin LS, Oliver P, Cooks RG. Interactive hyperspectral approach for esploring and interpreting DESI-MS images of cancerous and normal tissue section. Analyst. 2012. https://doi.org/10.1039/c2an35122f.
Eberlin LS, Dill AL, Golby AJ, Ligon KL, Wiseman JM, Cooks RG, et al. Discrimination of human astrocytoma subtypes by lipid analysis using desorption electrospray ionization imaging mass spectrometry. Angew Chem Int Ed. 2010. https://doi.org/10.1002/anie.201001452.
Dill AL, Eberlin LS, Zheng C, Costa AB, Ifa DR, Cheng L, et al. Multivariate statistical differentiation of renal cell carcinomas based on lipidomic analysis by ambient ionization imaging mass spectrometry. Anal Bioanal Chem. 2010. https://doi.org/10.1007/s00216-010-4259-6.
Eberlin LS, Norton I, Dill AL, Golby AJ, Ligon KL, Santagata S, et al. Classifying human brain tumors by lipid imaging with mass spectrometry. Cancer Res. 2012. https://doi.org/10.1158/0008-5472.CAN-11-2465.
Masterson TA, Dill AL, Eberlin LS, Mattarozzi M, Cheng L, Beck SD, et al. Distinctive glycerophospholipid profiles of human seminoma and adjacent normal tissues by desorption electrospray ionization imaging mass spectrometry. J Am Soc Mass Spectrom. 2011. https://doi.org/10.1007/s13361-011-0134-8.
Sans M, Gharpure K, Tibshirani R, Zhang J, Liang L, Liu J, Young JH, Dood RL, Sood AK, Eberlin LS. Metabolic markers and statistical prediction of serous ovarian cancer aggressiveness by ambient ionization mass spectrometry imaging. Cancer Res. 2017;77(11):2903–2913. https://doi.org/10.1158/0008-5472.CAN-16-3044.
Campbell DI, Ferreira CR, Eberlin LS, Cooks RG. Improved spatial resolution in the imaging of biological tissue using desorption electrospray ionization. Anal Bioanal Chem. 2012. https://doi.org/10.1007/s00216-012-6173-6.
Maillard V, Desmarchais A, Durcin M, Uzbekova S, Elis S. Docosahexaenoic acid(DHA) effects on proliferation and steroidogenesis of bovine granulosa cells. Reprod Biol Endocrinol. 2018;16(1):40. https://doi.org/10.1186/s12958-018-0357-7.
Uzbekova S, Elis S, Teixeira-Gomes AP, Desmarchais A, Maillard V, Labas V. MALDI mass spectrometry imaging of lipids and gene expression reveals differences in fatty acid metabolism between follicular compartments in porcine ovaries. Biology (Basel). 2015. https://doi.org/10.3390/biology4010216.
Pirro V, Oliveri P, Ferreira CR, González-Serrano AF, Machaty Z, Cooks RG. Lipid characterization of individual porcine oocytes by dual mode DESI-MS and data fusion. Anal Chim Acta. 2014. https://doi.org/10.1016/j.aca.2014.08.001.
Tata A, Sudano MJ, Santos VG, Landim-Alvarenga FD, Ferreira CR, Eberlin MN. Optimal single-embryo mass spectrometry fingerprinting. J Mass Spectrom. 2013. https://doi.org/10.1002/jms.3231.
Houmard BS, Guan Z, Stokes BT, Ottobre JS. Activation of elements of the phosphatidylinositol pathway in the primate corpus luteum by prostaglandin E2. Mol Hum Reprod. 1996;2(11):829–34.
Kato S, Shiratsuchi A, Nagaosa K, Nakanishi Y. Phosphatidylserine- and integrin-mediated phagocytosis of apoptotic luteal cells by macrophages of the rat. Develop Growth Differ. 2005;47(3):153–61.
Lebars H, Thibault C. Comparative aspects of reproduction in domestic animals. Therapie. 1964;19:1145–77.
Driancourt M. Regulation of ovarian follicular dynamics in farm animals. Implications for manipulation of reproduction. Theriogenology. 2001. https://doi.org/10.1016/S0093-691X(01)00479-4.
Ginther OJ, Beg MA, Bergfelt DR, Donadeu FX, Kot K. Follicle selection in monovular species. Biol Reprod. 2001. https://doi.org/10.1095/biolreprod65.3.638.
Reynaud K, Halter S, Tahir Z, Thoumire S, Chebrout M, Chastant-Maillard S. Polyovular follicles. Gynecol Obstet Fertil. 2010. https://doi.org/10.1016/j.gyobfe.2010.04.008.
Vanholder T, Leroy JL, Soom AV, Opsomer G, Maes D, Coryn M, et al. Effect of non-esterified fatty acids on bovine granulosa cell steroidogenesis and proliferation in vitro. Anim Reprod Sci. 2005. https://doi.org/10.1016/j.anireprosci.2004.09.006.
Soede NM, Langendijk P, Kemp B. Reproductive cycles in pigs. Anim Reprod Sci. 2011. https://doi.org/10.1016/j.anireprosci.2011.02.025.
Kemiläinen H, Adam M, Mäki-Jouppila J, Damdimopoulou P, Damdimopoulos AE, Kere J, et al. The hydroxysteroid (17β) dehydrogenase family gene HSD17B12 is involved in the prostaglandin synthesis pathway, the ovarian function, and regulation of fertility. Endocrinology. 2016. https://doi.org/10.1210/en.2016-1252.
Downey BR, Mootoo JE, Doyle SE. A role for lipoxygenase metabolites of arachidonic acid in porcine ovulation. Anim Reprod Sci. 1998. https://doi.org/10.1016/S0378-4320(97)00080-8.
Skarzynski DJ, Piotrowska-Tomala KK, Lukasik K, Galvão A, Farberov S, Zalman Y, et al. Growth and regression in bovine corpora lutea: regulation by local survival and death pathways. Reprod Domest Anim. 2013. https://doi.org/10.1111/rda.12203.
Murphy BD, Gévry N, Ruiz-Cortés T, Coté F, Downey BR, Sirois J. Formation and early development of the corpus luteum in pigs. Reprod Suppl. 2001;58:47–63.
Hinckley T Sr, Clark RM, Bushmich SL, Milvae RA. Long chain polyunsaturated fatty acids and bovine luteal cell function. Biol Reprod. 1996;55(2):445–9.
Oktem O, Urnam B. Understanding follicle growth in vivo. Hum Reprod. 2010. https://doi.org/10.1093/humrep/deq275.
Wonnacott KE, Kwong WY, Hughes J, Salter AM, Lea RG, Garnsworthy PC, et al. Dietary omega-3 and -6 polyunsatured fatty acids affect the composition and developmental of sheep granulosa cells, oocytes and embryos. Reproduction. 2010;139(1):57–6.
Acknowledgments
We thank Dr. Francesca E. Duncan for her valuable comments on an initial draft of this manuscript.
Funding
This study received funding from Grant No. UL1TR002529 (A. Shekhar, PI) from the National Institutes of Health, National Center for Advancing Translational Sciences, Clinical, and Translational Sciences Award; from the National Institute of Biomedical Imaging and Bioengineering, NIH Grant R21EB015722; from the National Institute of Allergy and Infectious Diseases, NIH Grant R01AI122298; and from the Purdue University Center for Cancer Research Small Grants Program.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The present study has used mice samples from the Purdue University Center for Cancer Research’s Transgenic Mouse Core Facility. The study received approval from the Purdue Animal Care and Use Committee (protocol 11-060).
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 2790 kb)
Rights and permissions
About this article
Cite this article
Cordeiro, F.B., Jarmusch, A.K., León, M. et al. Mammalian ovarian lipid distributions by desorption electrospray ionization–mass spectrometry (DESI-MS) imaging. Anal Bioanal Chem 412, 1251–1262 (2020). https://doi.org/10.1007/s00216-019-02352-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02352-6