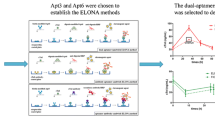
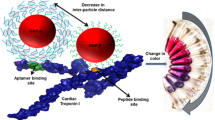
Abstract
Early diagnosis of acute myocardial infarction (AMI) is of outmost importance to reduce the mortality rate, and cardiac troponins are considered the gold standard biomarkers of myocardial necrosis. In this scenario, the characterization of two troponin T (TnT)-binding aptamers as viable alternative to antibodies employed on clinical immunoassays is here reported for the first time. Their recognition ability was first investigated through surface plasmon resonance (SPR). Subsequently, an enzyme-linked oligonucleotide assay (ELONA) was developed on common 96-well polystyrene plates, both by direct and sandwich detection strategies for comparison. In both cases, the assay exhibits a detection ability of TnT in the range of low nanomolar but a great advantage on serum interference was obtained by using both aptamers in a sandwich format, with excellent reproducibility and recovery values. Despite the sensitivity needing to be enhanced to the low picomolar range, these results are encouraging for the development of new, low-cost, and rapid antibody-free colorimetric assays for AMI studies based on aptamer–Troponin T recognition.




Similar content being viewed by others
References
Palladino P, Minunni M, Scarano S. Cardiac troponin T capture and detection in real-time via epitope-imprinted polymer and optical biosensing. Biosens Bioelectron. 2018. https://doi.org/10.1016/j.bios.2018.01.068.
Chen L, Wang X, Lu W, Wu X, Li J. Molecular imprinting: perspectives and applications. Chem Soc Rev. 2016. https://doi.org/10.1039/c6cs00061d.
Justino CIL, Duarte AC, Rocha-Santos TAP. Analytical applications of affibodies. Trends Anal Chem. 2015. https://doi.org/10.1016/j.trac.2014.10.014.
Zhou W, Huang PJJ, Ding J, Liu J. Aptamer-based biosensors for biomedical diagnostics. Analyst. 2014. https://doi.org/10.1039/c4an00132j.
Hussain M, Wackerlig J, Lieberzeit PA. Biomimetic strategies for sensing biological species. Biosensors. 2013. https://doi.org/10.3390/bios3010089.
Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990. https://doi.org/10.1038/346818a0.
Stoltenburg R, Krafčiková P, Víglaský V, Strehlitz B. G-quadruplex aptamer targeting Protein A and its capability to detect Staphylococcus aureus demonstrated by ELONA. Sci Rep. 2016. https://doi.org/10.1038/srep33812.
Scarano S, Dausse E, Crispo F, Toulmé JJ, Minunni M. Design of a dual aptamer-based recognition strategy for human matrix metalloproteinase 9 protein by piezoelectric biosensors. Anal Chim Acta. 2015. https://doi.org/10.1016/j.aca.2015.07.009.
Lamberti I, Scarano S, Esposito CL, Antoccia A, Antonini G, Tanzarella C, et al. In vitro selection of RNA aptamers against CA125 tumor marker in ovarian cancer and its study by optical biosensing. Methods. 2016. https://doi.org/10.1016/j.ymeth.2015.10.022.
Nie J, Deng Y, Deng QP, Zhang DW, Zhou YL, Zhang XX. A self-assemble aptamer fragment/target complex based high-throughput colorimetric aptasensor using enzyme linked aptamer assay. Talanta. 2013. https://doi.org/10.1016/j.talanta.2012.11.018.
Lisi S, Fiore E, Scarano S, Pascale E, Boehman Y, Ducongé F, et al. Non-SELEX isolation of DNA aptamers for the homogeneous-phase fluorescence anisotropy sensing of tau proteins. Anal Chim Acta. 2018. https://doi.org/10.1016/j.aca.2018.07.029.
Bunka DH, Stockley PG. Aptamers come of age - at last. Nat Rev Microbiol. 2006. https://doi.org/10.1038/nrmicro1458.
Niazi S, Wang X, Pasha I, Khan IM, Zhao S, Shoaib M, et al. A novel bioassay based on aptamer-functionalized magnetic nanoparticle for the detection of zearalenone using time resolved-fluorescence NaYF4: Ce/Tb nanoparticles as signal probe. Talanta. 2018. https://doi.org/10.1016/j.talanta.2018.04.013.
Jeong S, Paeng IR. Sensitivity and selectivity on aptamer-based assay: the determination of tetracycline residue in bovine milk. Sci World J. 2012. https://doi.org/10.1100/2012/159456.
Drolet DW, Moon-McDermott L, Romig TS. An enzyme-linked oligonucleotide assay. Nat Biotechnol. 1996. https://doi.org/10.1038/nbt0896-1021.
Sypabekova M, Bekmurzayeva A, Wang R, Li Y, Nogues C, Kanayeva D. Selection, characterization, and application of DNA aptamers for detection of Mycobacterium tuberculosis secreted protein MPT64. Tuberculosis. 2017. https://doi.org/10.1016/j.tube.2017.03.004.
Xu J, Zhang X, Shuanghai Z, Shen J, Yang D, Wu J, et al. A DNA aptamer efficiently inhibits the infectivity of Bovine herpesvirus 1 by blocking viral entry. Sci Rep. 2017. https://doi.org/10.1038/s41598-017-10070-1.
Li HH, Wen CY, Hong CY, Lai JC. Evaluation of aptamer specificity with or without primers using clinical samples for C-reactive protein by magnetic-assisted rapid aptamer selection. RSC Adv. 2017. https://doi.org/10.1039/c7ra07249j.
Le TT, Adamiak B, Benton DJ, Johnson CJ, Sharma S, Fenton R, et al. Aptamer-based biosensors for the rapid visual detection of flu viruses. Chem Commun. 2014. https://doi.org/10.1039/c4cc07888h.
Barthelmebs L, Jonca J, Hayat A, Prieto-Simon B, Marty JL. Enzyme-linked aptamer assays (ELAAs), based on a competition format for a rapid and sensitive detection of Ochratoxin A in wine. Food Control. 2011. https://doi.org/10.1016/j.foodcont.2010.11.005.
García-Recio EM, Pinto-Díez C, Pérez-Morgado MI, García-Hernández M, Fernández G, Martín ME, González VM. Characterization of MNK1b DNA aptamers that inhibit proliferation in MDA-MB231 breast cancer cells. Mol Ther Nucleic Acids 2016; https://doi.org/10.1038/mtna.2015.50.
Park H, Paeng IR. Development of direct competitive enzyme-linked aptamer assay for determination of dopamine in serum. Anal Chim Acta. 2011. https://doi.org/10.1016/j.aca.2010.11.010.
Reichlin T, Hochholzer W, Bassetti S, Steuer S, Stelzig C, Hartwiger S, et al. Early diagnosis of myocardial infarction with sensitive cardiac troponin assays. N Engl J Med. 2009. https://doi.org/10.1056/NEJMoa0900428.
White HD, Chew DP. Acute myocardial infarction. Lancet. 2008; https://doi.org/10.1016/S0140-6736(08)61237-4.
Westermann D, Neumann JT, Sörensen NA, Blankenberg S. High-sensitivity assays for troponin in patients with cardiac disease. Nat Rev Cardiol. 2017. https://doi.org/10.1038/nrcardio.2017.48.
Korff S, Katus HA, Giannitsis E. Differential diagnosis of elevated troponins. Heart. 2006. https://doi.org/10.1136/hrt.2005.071282.
Daubert MA, Jeremias A. The utility of troponin measurement to detect myocardial infarction: review of the current findings. Vasc Health Risk Manag. 2010. https://doi.org/10.2147/VHRM.S5306.
Ibanez B, James S, Agewall S, et al. ESC guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J. 2017. https://doi.org/10.1093/eurheartj/ehx393.
Lippi G. Novel troponin immunoassay for early ACS rule-out. Nat Rev Cardiol. 2016. https://doi.org/10.1038/nrcardio.2015.174.
Drescher DG, Ramakrishnan NA, Drescher MJ. Surface plasmon resonance (SPR) analysis of binding interactions of proteins in inner-ear sensory epithelia. Methods Mol Biol. 2009. https://doi.org/10.1007/978-1-59745-523-7_20.
Palladino P, Scaglione GL, Arcovito A, Vitale RM, Amodeo P, Vallone B, et al. Neuroglobin–prion protein interaction: what’s the function? J Pept Sci. 2011. https://doi.org/10.1002/psc.1333.
Palladino P, Aura AM, Spoto G. Surface plasmon resonance for the label-free detection of Alzheimer’s β-amyloid peptide aggregation. Anal Bioanal Chem. 2016. https://doi.org/10.1007/s00216-015-9172-6.
D’Agata R, Palladino P, Spoto G. Streptavidin-coated gold nanoparticles: critical role of oligonucleotides on stability and fractal aggregation. Beilstein J Nanotechnol. 2017. https://doi.org/10.3762/bjnano.8.1.
Katrukha IA. Human cardiac troponin complex. Structure and functions. Biochemistry. 2013. https://doi.org/10.1134/S0006297913130063.
Takeda S, Yamashita A, Maeda K, Maéda Y. Structure of the core domain of human cardiac troponin in the Ca2+-saturated form. Nature. 2003. https://doi.org/10.1038/nature01780.
Centi S, Tombelli S, Minunni M, Mascini M. Aptamer-based detection of plasma proteins by an electrochemical assay coupled to magnetic beads. Anal Chem. 2007. https://doi.org/10.1021/ac061879p.
Liotta LA, Espina V, Mehta AI, Calvert V, Rosenblatt K, Geho D, et al. Protein microarrays: meeting analytical challenges for clinical applications. Cancer Cell. 2003. https://doi.org/10.1016/S1535-6108(03)00086-2.
Espina V, Woodhouse EC, Wulfkuhle J, Asmussen HD, Petricoin EF III, Liotta LA. Protein microarray detection strategies: focus on direct detection technologies. J Immunol Methods. 2004. https://doi.org/10.1016/j.jim.2004.04.013.
Goddard JM, Hotchkiss JH. Polymer surface modification for the attachment of bioactive compounds. Prog Polym Sci. 2007. https://doi.org/10.1016/j.progpolymsci.2007.04.002.
Acknowledgments
Simona Scarano thanks the Ministry of Education, University and Research (MIUR) for the scientific program SIR2014 Scientific Independence of young Researchers (RBSI1455LK), ‘Early diagnosis of acute myocardial infarction by nanosensing: coupling emerging bioreceptors for Troponin T to Localized Surface Plasmon Resonance (LSPR) for a high sensitive point-of-care testing’.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Published in the topical collection New Developments in Biosensors with guest editors Francesco Baldini and Maria Minunni.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Torrini, F., Palladino, P., Brittoli, A. et al. Characterization of troponin T binding aptamers for an innovative enzyme-linked oligonucleotide assay (ELONA). Anal Bioanal Chem 411, 7709–7716 (2019). https://doi.org/10.1007/s00216-019-02014-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-02014-7