Abstract
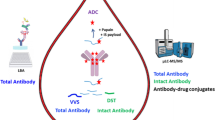
Pharmacokinetic analysis of antibody-drug conjugates (ADCs) requires characterization and quantification of both the antibody-conjugated cytotoxic drug molecule (acDrug) as well as the antibody vehicle, among other analytes, in order to assess the safety and efficacy of ADCs. Due to the complexity of biological matrices, immunoaffinity capture is widely used for enrichment of the biotherapeutic, followed by enzymatic or chemical release of the drug and LC-MS/MS analysis to provide the concentration of acDrug. This bioanalytical strategy has been used successfully with ADCs, but is limited to ADCs having cleavable linkers. Herein, we developed a sensitive and specific method that involved subjecting the ADC to tryptic digestion, and measured a peptide that included cysteine conjugated to the drug to provide quantification of acDrug. Using this method for a THIOMAB™ antibody-drug conjugate (TDC) conjugated to MMAE via a cleavable linker, valine–citrulline, we compared peptide-linker MMAE data from the new assay format with earlier MMAE data for acDrug. This showed that the new assay format provides robust acDrug as well as total antibody concentration to study in vitro stability of the TDC in multiple matrices and in vivo pharmacokinetic models of TDC in rat and mouse. The data from the two orthogonal modes of acDrug analysis showed good agreement with each other, allowing us to successfully quantify acDrug to study the stability in vitro and the pharmacokinetic parameters in vivo. This new assay strategy allows acDrug quantification for ADCs with non-cleavable linkers where the resulting acDrug analyte is a peptide-linker drug.





Similar content being viewed by others
References
Polakis P. Antibody drug conjugates for cancer therapy. Pharmacol Rev. 2016;68:3–19. https://doi.org/10.1124/pr.114.009373.
Peters C, Brown S. Antibody-drug conjugates as novel anti-cancer chemotherapeutics. Biosci Rep. 2015;35. https://doi.org/10.1042/BSR20150089.
Junutula JR, Raab H, Clark S, Bhakta S, Leipold DD, Weir S, et al. Site-specific conjugation of a cytotoxic drug to an antibody improves the therapeutic index. Nat Biotechnol. 2008;26:925–32. https://doi.org/10.1038/nbt.1480.
Strop P, Delaria K, Foletti D, Witt JM, Hasa-Moreno A, Poulsen K, et al. Site-specific conjugation improves therapeutic index of antibody drug conjugates with high drug loading. Nat Biotechnol. 2015;33:694–6. https://doi.org/10.1038/nbt.3274.
Panowski S, Bhakta S, Raab H, Polakis P, Junutula JR. Site-specific antibody drug conjugates for cancer therapy. mAbs. 2014;6:34–45. https://doi.org/10.4161/mabs.27022.
Axup JY, Bajjuri KM, Ritland M, Hutchins BM, Kim CH, Kazane SA, et al. Synthesis of site-specific antibody-drug conjugates using unnatural amino acids. Proc Natl Acad Sci U S A. 2012;109:16101–6. https://doi.org/10.1073/pnas.1211023109.
Tian F, Lu Y, Manibusan A, Sellers A, Tran H, Sun Y, et al. A general approach to site-specific antibody drug conjugates. Proc Natl Acad Sci U S A. 2014;111:1766–71. https://doi.org/10.1073/pnas.1321237111.
Strop P, Liu S-H, Dorywalska M, Delaria K, Dushin RG, Tran T-T, et al. Location matters: site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem Biol. 2013;20:161–7. https://doi.org/10.1016/j.chembiol.2013.01.010.
Thompson P, Ezeadi E, Hutchinson I, Fleming R, Bezabeh B, Lin J, et al. Straightforward glycoengineering approach to site-specific antibody-pyrrolobenzodiazepine conjugates. ACS Med Chem Lett. 2016;7:1005–8. https://doi.org/10.1021/acsmedchemlett.6b00278.
Beerli RR, Hell T, Merkel AS, Grawunder U. Sortase enzyme-mediated generation of site-specifically conjugated antibody drug conjugates with high in vitro and in vivo potency. PLoS One. 2015;10:e0131177. https://doi.org/10.1371/journal.pone.0131177.
Dorywalska M, Strop P, Melton-Witt JA, Hasa-Moreno A, Farias SE, Galindo Casas M, et al. Effect of attachment site on stability of cleavable antibody drug conjugates. Bioconjug Chem. 2015;26:650–9. https://doi.org/10.1021/bc5005747.
Shen B-Q, Xu K, Liu L, Raab H, Bhakta S, Kenrick M, et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat Biotechnol. 2012;30:184–9. https://doi.org/10.1038/nbt.2108.
Kaur S, Xu K, Saad OM, Dere RC, Carrasco-Triguero M. Bioanalytical assay strategies for the development of antibody–drug conjugate biotherapeutics. Bioanalysis. 2013;5:201–26. https://doi.org/10.4155/bio.12.299.
Gorovits B, Alley SC, Bilic S, Booth B, Kaur S, Oldfield P, et al. Bioanalysis of antibody–drug conjugates: American Association of Pharmaceutical Scientists Antibody–Drug Conjugate Working Group position paper. Bioanalysis. 2013;5:997–1006. https://doi.org/10.4155/bio.13.38.
Xu K, Liu L, Maia M, Li J, Lowe J, Song A, et al. A multiplexed hybrid LC-MS/MS pharmacokinetic assay to measure two co-administered monoclonal antibodies in a clinical study. Bioanalysis. 2014;6:1781–94. https://doi.org/10.4155/bio.14.142.
Li H, Ortiz R, Tran L, Hall M, Spahr C, Walker K, et al. General LC-MS/MS method approach to quantify therapeutic monoclonal antibodies using a common whole antibody internal standard with application to preclinical studies. Anal Chem. 2012;84:1267–73. https://doi.org/10.1021/ac202792n.
Lu Q, Zheng X, McIntosh T, Davis H, Nemeth JF, Pendley C, et al. Development of different analysis platforms with LC−MS for pharmacokinetic studies of protein drugs. Anal Chem. 2009;81:8715–23. https://doi.org/10.1021/ac901991x.
Kaur S, Liu L, Cortes DF, Shao J, Jenkins R, Mylott WR, et al. Validation of a biotherapeutic immunoaffinity-LC-MS/MS assay in monkey serum: “plug-and-play” across seven molecules. Bioanalysis. 2016;8:1565–77. https://doi.org/10.4155/bio-2016-0117.
Rago B, Tumey LN, Wei C, Barletta F, Clark T, Hansel S, et al. Quantitative conjugated payload measurement using enzymatic release of antibody-drug conjugate with cleavable linker. Bioconjug Chem. 2017;28:620–6. https://doi.org/10.1021/acs.bioconjchem.6b00695.
Sanderson RJ, Nicholas ND, Baker Lee C, Hengel SM, Lyon RP, Benjamin DR, et al. Antibody-conjugated drug assay for protease-cleavable antibody-drug conjugates. Bioanalysis. 2016;8:55–63. https://doi.org/10.4155/bio.15.230.
Xu L, Packer LE, Li C, Abdul-Hadi K, Veiby P. A generic approach for simultaneous measurements of total antibody and cleavable antibody-conjugated drug by LC/MS/MS. Anal Biochem. 2017;537:33–6. https://doi.org/10.1016/j.ab.2017.08.024.
Sanderson RJ, Hering MA, James SF, Sun MMC, Doronina SO, Siadak AW, et al. In vivo drug-linker stability of an anti-CD30 dipeptide-linked auristatin immunoconjugate. Clin Cancer Res Off J Am Assoc Cancer Res. 2005;11:843–52.
Klussman K, Mixan BJ, Cerveny CG, Meyer DL, Senter PD, Wahl AF. Secondary mAb--vcMMAE conjugates are highly sensitive reporters of antibody internalization via the lysosome pathway. Bioconjug Chem. 2004;15:765–73. https://doi.org/10.1021/bc049969t.
Chelius D, Rehder DS, Bondarenko PV. Identification and characterization of deamidation sites in the conserved regions of human immunoglobulin gamma antibodies. Anal Chem. 2005;77:6004–11. https://doi.org/10.1021/ac050672d.
Pace AL, Wong RL, Zhang YT, Kao Y-H, Wang YJ. Asparagine deamidation dependence on buffer type, pH, and temperature. J Pharm Sci. 2013;102:1712–23. https://doi.org/10.1002/jps.23529.
Hao P, Ren Y, Alpert AJ, Sze SK. Detection, evaluation and minimization of nonenzymatic deamidation in proteomic sample preparation. Mol Cell Proteomics MCP. 2011;10:O111.009381. https://doi.org/10.1074/mcp.O111.009381.
Chelius D, Jing K, Lueras A, Rehder DS, Dillon TM, Vizel A, et al. Formation of pyroglutamic acid from N-terminal glutamic acid in immunoglobulin gamma antibodies. Anal Chem. 2006;78:2370–6. https://doi.org/10.1021/ac051827k.
Yu L, Vizel A, Huff MB, Young M, Remmele RL, He B. Investigation of N-terminal glutamate cyclization of recombinant monoclonal antibody in formulation development. J Pharm Biomed Anal. 2006;42:455–63. https://doi.org/10.1016/j.jpba.2006.05.008.
Xu K, Liu L, Dere R, Mai E, Erickson R, Hendricks A, et al. Characterization of the drug-to-antibody ratio distribution for antibody–drug conjugates in plasma/serum. Bioanalysis. 2013;5:1057–71. https://doi.org/10.4155/bio.13.66.
Acknowledgements
The authors would like to thank Michelle Schweiger, Nina Ljumanovic, and Roxanne Andaya for their support in conduct of animal studies. Additionally, Patricia Siguenza is acknowledged for reviewing the paper.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The authors state that they have obtained appropriate Institutional Review Board approval or have followed the principles outlined in the Declaration of Helsinki for all animal experimental investigations.
Conflict of interest
All of the authors are employees of Genentech. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 744 kb)
Rights and permissions
About this article
Cite this article
Hyung, SJ., Li, D., Koppada, N. et al. Method development of a novel PK assay for antibody-conjugated drug measurement of ADCs using peptide-linker drug analyte. Anal Bioanal Chem 411, 2587–2596 (2019). https://doi.org/10.1007/s00216-019-01701-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01701-9