Abstract
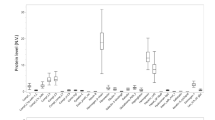
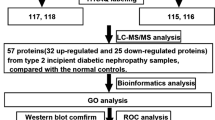
Coronary artery disease (CAD) is a manifestation of systemic atherosclerotic disease. It is assessed by intervention or traditional scoring risk factors. Diagnosis is limited by inaccurate and invasive methods. Developing noninvasive methods to screen for the risk of CAD is a major challenge. We aimed to identify urinary proteins associated with CAD. We utilized iTRAQ labeling followed by 2D LC-MS/MS to compare the urinary proteome of CAD patients to healthy cohorts. The multiple reaction monitoring (MRM) was used to verify the differential proteins. ROC analysis based on MRM data was used to evaluate the diagnostic application. A total of 876 proteins were quantified, and 100 differential proteins were found. Functional analysis revealed that the differential proteins were mainly associated with Liver X Receptor/Retinoid X Receptor (LXR/RXR) pathway activation, atherosclerosis signaling, production of nitric oxide and reactive oxygen species, and the top upstream regulator of the differential proteins by IPA analysis indicated to the APOE. Nineteen differential proteins were verified by MRM analysis. ROC based on MRM data revealed that the combination of two proteins (APOD and TFF1) could diagnose CAD with 85% sensitivity and 99% specificity (AUC 0.95). The urinary proteome might reflect the pathophysiological changes in CAD and be used for the clinical study of CAD.





Similar content being viewed by others
References
Weber C, Noels H. Atherosclerosis: current pathogenesis and therapeutic options. Nat Med. 2011;17(11):1410–22.
Karabay CY, Kocabay G, Guler A, Kalayci A, Akgun T, Kirma C. Clinical usefulness of carotid ultrasonography in patients with an inconclusive exercise treadmill stress test result. J Cardiol. 2014;64(1):70–4.
Patel AK, Suri HS, Singh J, Kumar D, Shafique S, Nicolaides A, et al. A review on atherosclerotic biology, wall stiffness, physics of elasticity, and its ultrasound-based measurement. Curr Atheroscler Rep. 2016;18(12):83.
Honda S, Kataoka Y, Kanaya T, Noguchi T, Ogawa H, Yasuda S. Characterization of coronary atherosclerosis by intravascular imaging modalities. Cardiovasc Diagn Ther. 2016;6(4):368–81.
Libby P, Ridker PM, Hansson GK. Progress and challenges in translating the biology of atherosclerosis. Nature. 2011;473(7347):317–25.
Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–97.
Rossing K, Bosselmann HS, Gustafsson F, Zhang ZY, Gu YM, Kuznetsova T, et al. Urinary proteomics pilot study for biomarker discovery and diagnosis in heart failure with reduced ejection fraction. PLoS One. 2016;11(6):e0157167.
An M, Gao Y. Urinary biomarkers of brain diseases. Genomics Proteomics Bioinformatics. 2015;13(6):345–54.
Zimmerli LU, Schiffer E, Zurbig P, Good DM, Kellmann M, Mouls L, et al. Urinary proteomic biomarkers in coronary artery disease. Mol Cell Proteomics. 2008;7(2):290–8.
Von Zur Muhlen C, Schiffer E, Zuerbig P, Kellmann M, Brasse M, Meert N, et al. Evaluation of urine proteome pattern analysis for its potential to reflect coronary artery atherosclerosis in symptomatic patients. J Proteome Res. 2009;8(1):335–45.
Delles C, Schiffer E, von Zur Muhlen C, Peter K, Rossing P, Parving HH, et al. Urinary proteomic diagnosis of coronary artery disease: identification and clinical validation in 623 individuals. J Hypertens. 2010;28(11):2316–22.
Von zur Muhlen C, Schiffer E, Sackmann C, Zurbig P, Neudorfer I, Zirlik A, et al. Urine proteome analysis reflects atherosclerotic disease in an ApoE−/− mouse model and allows the discovery of new candidate biomarkers in mouse and human atherosclerosis. Mol Cell Proteomics. 2012;11(7):M111 013847.
Delles C, Diez J, Dominiczak AF. Urinary proteomics in cardiovascular disease: achievements, limits and hopes. Proteomics Clin Appl. 2011;5(5–6):222–32.
Raimondo F, Corbetta S, Morosi L, Chinello C, Gianazza E, Castoldi G, et al. Urinary exosomes and diabetic nephropathy: a proteomic approach. Mol BioSyst. 2013;9(6):1139–46.
Gajbhiye A, Dabhi R, Taunk K, Vannuruswamy G, RoyChoudhury S, Adhav R, et al. Urinary proteome alterations in HER2 enriched breast cancer revealed by multipronged quantitative proteomics. Proteomics. 2016;16(17):2403–18.
Yu Y, Sikorski P, Bowman-Gholston C, Cacciabeve N, Nelson KE, Pieper R. Diagnosing inflammation and infection in the urinary system via proteomics. J Transl Med. 2015;13:111.
Husi H, Skipworth RJ, Cronshaw A, Fearon KC, Ross JA. Proteomic identification of potential cancer markers in human urine using subtractive analysis. Int J Oncol. 2016;48(5):1921–32.
Sun W, Gao S, Wang L, Chen Y, Wu S, Wang X, et al. Microwave-assisted protein preparation and enzymatic digestion in proteomics. Mol Cell Proteomics. 2006;5(4):769–76.
Guo Z, Cheng J, Sun H, Sun W. A qualitative and quantitative evaluation of the peptide characteristics of microwave- and ultrasound-assisted digestion in discovery and targeted proteomic analyses. Rapid Commun Mass Spectrom. 2017;31(16):1353–62.
Robin X, Turck N, Hainard A, Tiberti N, Lisacek F, Sanchez JC, et al. pROC: an open-source package for R and S+ to analyze and compare ROC curves. BMC Bioinformatics. 2011;12:77.
Youden WJ. Index for rating diagnostic tests. Cancer. 1950;3(1):32–5.
Marimuthu A, O'Meally RN, Chaerkady R, Subbannayya Y, Nanjappa V, Kumar P, et al. A comprehensive map of the human urinary proteome. J Proteome Res. 2011;10(6):2734–43.
Kietselaer BL, Reutelingsperger CP, Heidendal GA, Daemen MJ, Mess WH, Hofstra L, et al. Noninvasive detection of plaque instability with use of radiolabeled annexin A5 in patients with carotid-artery atherosclerosis. N Engl J Med. 2004;350(14):1472–3.
Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler Thromb. 1994;14(1):133–40.
Perdomo G, Henry Dong H. Apolipoprotein D in lipid metabolism and its functional implication in atherosclerosis and aging. Aging. 2009;1(1):17–27.
Tsukamoto K, Mani DR, Shi J, Zhang S, Haagensen DE, Otsuka F, et al. Identification of apolipoprotein D as a cardioprotective gene using a mouse model of lethal atherosclerotic coronary artery disease. Proc Natl Acad Sci U S A. 2013;110(42):17023–8.
Masiakowski P, Breathnach R, Bloch J, Gannon F, Krust A, Chambon P. Cloning of cDNA sequences of hormone-regulated genes from the MCF-7 human breast cancer cell line. Nucleic Acids Res. 1982;10(24):7895–903.
Ribieras S, Tomasetto C, Rio MC. The pS2/TFF1 trefoil factor, from basic research to clinical applications. Biochim Biophys Acta. 1998;1378(1):F61–77.
Aamann L, Vestergaard EM, Gronbaek H. Trefoil factors in inflammatory bowel disease. World J Gastroenterol. 2014;20(12):3223–30.
Kjellev S. The trefoil factor family - small peptides with multiple functionalities. Cell Mol Life Sci. 2009;66(8):1350–69.
Liu SQ, Tefft BJ, Roberts DT, Zhang LQ, Ren Y, Li YC, et al. Cardioprotective proteins upregulated in the liver in response to experimental myocardial ischemia. Am J Physiol Heart Circ Physiol. 2012;303(12):H1446–58.
Chutipongtanate S, Nakagawa Y, Sritippayawan S, Pittayamateekul J, Parichatikanond P, Westley BR, et al. Identification of human urinary trefoil factor 1 as a novel calcium oxalate crystal growth inhibitor. J Clin Invest. 2005;115(12):3613–22.
Fach EM, Garulacan LA, Gao J, Xiao Q, Storm SM, Dubaquie YP, et al. In vitro biomarker discovery for atherosclerosis by proteomics. Mol Cell Proteomics. 2004;3(12):1200–10.
Martin-Ventura JL, Duran MC, Blanco-Colio LM, Meilhac O, Leclercq A, Michel JB, et al. Identification by a differential proteomic approach of heat shock protein 27 as a potential marker of atherosclerosis. Circulation. 2004;110(15):2216–9.
Sung HJ, Ryang YS, Jang SW, Lee CW, Han KH, Ko J. Proteomic analysis of differential protein expression in atherosclerosis. Biomarkers. 2006;11(3):279–90.
Donahue MP, Rose K, Hochstrasser D, Vonderscher J, Grass P, Chibout SD, et al. Discovery of proteins related to coronary artery disease using industrial-scale proteomics analysis of pooled plasma. Am Heart J. 2006;152(3):478–85.
Padro T, Pena E, Garcia-Arguinzonis M, Llorente-Cortes V, Badimon L. Low-density lipoproteins impair migration of human coronary vascular smooth muscle cells and induce changes in the proteomic profile of myosin light chain. Cardiovasc Res. 2008;77(1):211–20.
Banfi C, Brioschi M, Marenzi G, De Metrio M, Camera M, Mussoni L, et al. Proteome of platelets in patients with coronary artery disease. Exp Hematol. 2010;38(5):341–50.
Luczak M, Formanowicz D, Pawliczak E, Wanic-Kossowska M, Wykretowicz A, Figlerowicz M. Chronic kidney disease-related atherosclerosis - proteomic studies of blood plasma. Proteome Sci. 2011;9:25.
de la Cuesta F, Alvarez-Llamas G, Maroto AS, Donado A, Zubiri I, Posada M, et al. A proteomic focus on the alterations occurring at the human atherosclerotic coronary intima. Mol Cell Proteomics. 2011;10(4):M110.003517.
Salgado-Somoza A, Teijeira-Fernandez E, Fernandez AL, Gonzalez-Juanatey JR, Eiras S. Changes in lipid transport-involved proteins of epicardial adipose tissue associated with coronary artery disease. Atherosclerosis. 2012;224(2):492–9.
Dela Cuesta F, Barderas MG, Calvo E, Zubiri I, Maroto AS, Darde VM, et al. Secretome analysis of atherosclerotic and non-atherosclerotic arteries reveals dynamic extracellular remodeling during pathogenesis. J Proteome. 2012;75(10):2960–71.
Poduri A, Bahl A, Talwar KK, Khullar M. Proteomic analysis of circulating human monocytes in coronary artery disease. Mol Cell Biochem. 2012;360(1–2):181–8.
Riwanto M, Rohrer L, Roschitzki B, Besler C, Mocharla P, Mueller M, et al. Altered activation of endothelial anti- and proapoptotic pathways by high-density lipoprotein from patients with coronary artery disease: role of high-density lipoprotein-proteome remodeling. Circulation. 2013;127(8):891–904.
Vangala RK, Ravindran V, Kamath K, Rao VS, Sridhara H. Novel network biomarkers profile based coronary artery disease risk stratification in Asian Indians. Adv Biomed Res. 2013;2:59.
Kristensen LP, Larsen MR, Mickley H, Saaby L, Diederichsen AC, Lambrechtsen J, et al. Plasma proteome profiling of atherosclerotic disease manifestations reveals elevated levels of the cytoskeletal protein vinculin. J Proteome. 2014;101:141–53.
Dela Cuesta F, Zubiri I, Maroto AS, Posada M, Padial LR, Vivanco F, et al. Deregulation of smooth muscle cell cytoskeleton within the human atherosclerotic coronary media layer. J Proteome. 2013;82:155–65.
Lee MY, Huang CH, Kuo CJ, Lin CL, Lai WT, Chiou SH. Clinical proteomics identifies urinary CD14 as a potential biomarker for diagnosis of stable coronary artery disease. PLoS One. 2015;10(2):e0117169.
Brown CE, McCarthy NS, Hughes AD, Sever P, Stalmach A, Mullen W, et al. Urinary proteomic biomarkers to predict cardiovascular events. Proteomics Clin Appl. 2015;9(5–6):610–7.
Amin M, Pushpakumar S, Muradashvili N, Kundu S, Tyagi SC, Sen U. Regulation and involvement of matrix metalloproteinases in vascular diseases. Front Biosci (Landmark Ed). 2016;21:89–118.
Fitzsimmons PJ, Forough R, Lawrence ME, Gantt DS, Rajab MH, Kim H, et al. Urinary levels of matrix metalloproteinase 9 and 2 and tissue inhibitor of matrix metalloproteinase in patients with coronary artery disease. Atherosclerosis. 2007;194(1):196–203.
Funding
This work was supported by National Basic Research Program of China (No. 2013CB530805, 2014CBA02005), National Key Research and Development Program of China (No. 2016 YFC 1306300,2018YFC0910202), Key Basic Research Program of the Ministry of Science and Technology of China (No. 2013FY114100), National Natural Science Foundation of China (No. 30970650, 31200614, 31400669, 81371515, 81170665, 81560121), Beijing Natural Science Foundation (No. 7173264, 7172076), Beijing cooperative construction project (No.110651103), Beijing Science Program for the Top Young (No.2015000021223TD04), Beijing Normal University (No.11100704), Peking Union Medical College Hospital (No.2016-2.27), CAMS Innovation Fund for Medical Sciences (2017-I2M-1-009), and Biologic Medicine Information Center of China, National Scientific Data Sharing Platform for Population and Health.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 2461 kb)
Rights and permissions
About this article
Cite this article
Sun, H., Wang, D., Liu, D. et al. Differential urinary proteins to diagnose coronary heart disease based on iTRAQ quantitative proteomics. Anal Bioanal Chem 411, 2273–2282 (2019). https://doi.org/10.1007/s00216-019-01668-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01668-7