Abstract
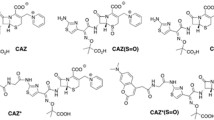
Multidrug-resistant bacteria are a great concern and a problem that must be addressed. Extended-spectrum β-lactamases are a common defence mechanism of bacteria to make β-lactam (BL) antibiotics ineffective. β-Lactamase inhibitors (BLIs) are consequently designed and are often clinically prescribed with a BL antibiotic to hinder degradation. Current studies focusing on how BL antibiotics or BLIs interact solely with the bacterial outer membrane nanopores (porins) on reaching the periplasmic side using a nanopore-based sensing technique. In electrochemical studies, the bias voltage allows real-time monitoring of BL antibiotics, BLIs and their mixture through the porin pathway at the single-molecule level. Here we consider the most abundant membrane protein from Escherichia coli (i.e. OmpF), purify and reconstitute the membrane protein in an artificial lipid bilayer and then study its ex vivo electrochemical behaviour. We show the piperacillin/tazobactam mixture interacts with OmpF, whereas the substrate interacts under the maximum bandwidth. The power spectrum analysis of the ionic current trace demonstrates the ampicillin/sulbactram mixture requires more energy than ampicillin alone to pass through the porin pathway. Our results demonstrate that clinically relevant combinations (e.g. piperacillin/tazobactam and ampicillin/sulbactam) interact more strongly with OmpF than either the BL antibiotic or the BLI alone. We suggest a quick and relatively cheap screening method to test the ability of BL antibiotics/BLIs to cross the bacterial cellular membrane.




Similar content being viewed by others
References
Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, et al. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis. 2009;48:1–2.
Centers for Disease Control and Prevention. Biggest threats and data. (2008). https://www.cdc.gov/drugresistance/biggest_threats.html.
European Centre for Disease Prevention and Control. (). https://ecdc.europa.eu/sites/portal/files/documents/AMR-surveillance-Europe-2016.
World Health Organization. Antibacterial agents in clinical development. (2017). http://www.who.int/medicines/areas/rational_use/antibacterial_agents_clinical_development/en/.
Bolla JM, Alibert-Franco S, Handzlik J, Chevalier J, Mahamoud A, Boyer G, et al. Strategies for bypassing the membrane barrier in multidrug resistant Gram-negative bacteria. FEBS Lett. 2011;585:1682–90.
Nikaido H. Molecular basis of bacterial outer membrane permeability revisited. Microbiol Mol Biol Rev. 2003;67:593–656.
Bi S, Yan Y, Hao S, Zhang S. Colorimetric logic gates based on supramolecular DNAzyme structures. Angew Chem Int Ed. 2010;122:4540–4.
Zhang XX, Song YZ, Fang F, Wu ZY. Sensitive paper-based analytical device for fast colorimetric detection of nitrite with smartphone. Anal Bioanal Chem. 2018;410:2665–9.
Guo W, Ding H, Gu C, Liu Y, Jiang X, Su B, et al. Potential-resolved multicolor electrochemiluminescence for multiplex immunoassay in a single sample. J Am Chem Soc. 2018;140:15904–15.
Hua X, Li H-W, Long Y-T. Investigation of silver nanoparticle induced lipids changes on a single cell surface by time-of-flight secondary ion mass spectrometry. Anal Chem. 2018;90:1072–6.
Prochnow H, Fetz V, Hotop SK, García-Rivera MA, Heumann A, Brönstrup M. Subcellular quantification of uptake in Gram-negative bacteria. Anal Chem. 2018. https://doi.org/10.1021/acs.analchem.8b03586.
Laxminarayan R, Matsoso P, Pant S, Brower C, Røttingen JA, Klugman K, et al. Access to effective antimicrobials: a worldwide challenge. Lancet. 2016;387:168–75.
Cowan SW, Schirmer T, Rummel G, Steiert M, Ghosh R, Pauptit RA, et al. Crystal structures explain functional properties of two E. coli porins. Nature. 1992;358:727–33.
Benz R, Schmid A, Vos-Scheperkeuter GH. Mechanism of sugar transport through the sugar-specific LamB channel of Escherichia coli outer membrane. J Membr Biol. 1987;100:21–9.
Stavenger RA, Winterhalter M. TRANSLOCATION project: how to get good drugs into bad bugs. Sci Transl Med. 2014;6:228ed7.
Wang J, Bafna JA, Bhamidimarri SP, Winterhalter M. Small molecule permeation across membrane channels: chemical modification to quantify transport across OmpF. Angew Chem Int Ed. 2019. https://doi.org/10.1002/anie.201814489.
Sheng Y, You Y, Cao Z, Liu L, Wu HC. Rapid and selective DNA-based detection of melamine using α-hemolysin nanopores. Analyst. 2018;143:2411–5.
Kasianowicz JJ, Brandin E, Branton D, Deamer DW. Characterization of individual polynucleotide molecules using a membrane channel. Proc Natl Acad Sci U S A. 1996;93:13770–3.
Cao C, Ying YL, Hu ZL, Liao DF, Tian H, Long YT. Discrimination of oligonucleotides of different lengths with a wild-type aerolysin nanopore. Nat Nanotechnol. 2016;11:713–8.
Cao C, Yu J, Wang YQ, Ying YL, Long YT. Driven translocation of polynucleotides through an aerolysin nanopore. Anal Chem. 2016;88:5046–9.
Stefureac R, Long YT, Kraatz HB, Howard P, Lee JS. Transport of α-helical peptides through α-hemolysin and aerolysin pores. Biochemistry. 2006;45:9172–9.
Wang HY, Ying YL, Li Y, Kraatz HB, Long YT. Nanopore analysis of β-amyloid peptide aggregation transition induced by small molecules. Anal Chem. 2011;83:1746–52.
Li S, Cao C, Yang J, Long YT. Detection of peptides with different charges and lengths by using the aerolysin nanopore. ChemElectroChem. 2018. https://doi.org/10.1002/celc.201800288.
Nikaido H. Outer membrane barrier as a mechanism of antimicrobial resistance. Antimicrob Agents Chemother. 1989;33:1831–6.
Drawz SM, Bonomo RA. Three decades of β-lactamase inhibitors. Clin Microbiol Rev. 2010;23:160–201.
Fluit AC, Jones ME, Schmitz FJ, Acar J, Gupta R, Verhoef J. Antimicrobial susceptibility and frequency of occurrence of clinical blood isolates in Europe from the SENTRY antimicrobial surveillance program, 1997 and 1998. Clin Infect Dis. 2000;30:454–60.
Acosta-Gutiérrez S, Ferrara L, Pathania M, Masi M, Wang J, Bodrenko I, et al. Getting drugs into Gram-negative bacteria: Rational rules for permeation through general porins. ACS Infect Dis. 2018;4:1487–98.
Thanassi DG, Suh G, Nikaido H. Role of outer membrane barrier in efflux-mediated tetracycline resistance of Escherichia coli. J Bacteriol. 1995;177:998–1007.
Sen K, Hellman J, Nikaido H. Porin channels in intact cells of Escherichia coli are not affected by Donnan potentials across the outer membrane. J Biol Chem. 1988;263:1182–7.
Ghai I, Winterhalter M, Wagner R. Probing transport of charged β-lactamase inhibitors through OmpC, a membrane channel from E. coli. Biochem Biophys Res Commun. 2017;484:51–5.
Ghai I, Pira A, Scorciapino MA, Bodrenko I, Benier L, Ceccarelli M, et al. General method to determine the flux of charged molecules through nanopores applied to β-lactamase inhibitors and OmpF. J Phys Chem Lett. 2017;8:1295–301.
Wang J, Benier L, Winterhalter M. Quantifying permeation of small charged molecules across channels: electrophysiology in small volumes. ACS Omega. 2018;3:17481–6.
Ionescu SA, Lee S, Housden NG, Kaminska R, Kleanthous C, Bayley H. Orientation of the OmpF porin in planar lipid bilayers. ChemBioChem. 2017;18:554–62.
Mahendran KR, Hajjar E, Mach T, Lovelle M, Kumar A, Sousa I, et al. Molecular basis of enrofloxacin translocation through OmpF, an outer membrane channel of Escherichia coli-when binding does not imply translocation. J Phys Chem B. 2010;114:5170–9.
Danelon C, Suenaga A, Winterhalter M, Yamato I. Molecular origin of the cation selectivity in OmpF porin: single channel conductances vs. free energy calculation. Biophys Chem. 2003;104:591–603.
Schwarz G, Danelon C, Winterhalter M. On translocation through a membrane channel via an internal binding site: kinetics and voltage dependence. Biophys J. 2003;84:2990–8.
Danelon C, Brando T, Winterhalter M. Probing the orientation of reconstituted maltoporin channels at the single-protein level. J Biol Chem. 2003;278:35542–51.
Mahendran KR, Singh PR, Arning J, Stolte S, Kleinekathöfer U, Winterhalter M. Permeation through nanochannels: revealing fast kinetics. J Phys Condens Matter. 2010;22:454131.
Bodrenko I, Bajaj H, Ruggerone P, Winterhalter M, Ceccarelli M. Analysis of fast channel blockage: revealing substrate binding in the microsecond range. Analyst. 2015;140:4820–7.
Bodrenko IV, Wang J, Salis S, Winterhalter M, Ceccarelli M. Sensing single molecule penetration into nanopores: pushing the time resolution to the diffusion limit. ACS Sens. 2017;2:1184–90.
Gu LQ, Cheley S, Bayley H. Electroosmotic enhancement of the binding of a neutral molecule to a transmembrane pore. Proc Natl Acad Sci U S A. 2003;100:15498–503.
Piguet F, Discala F, Breton MF, Pelta J, Bacri L, Oukhaled A. Electroosmosis through α-hemolysin that depends on alkali cation type. J Phys Chem Lett. 2014;5:4362–7.
Ghai I, Bajaj H, Bafna JA, Hussein HA, Winterhalter M, Wagner R. Ampicillin permeation across OmpF, the major outer-membrane channel in Escherichia coli. J Biol Chem. 2018;293:7030–7.
Zheng J, Yang R, Shi M, Wu C, Fang X, Li Y, et al. Rationally designed molecular beacons for bioanalytical and biomedical applications. Chem Soc Rev. 2015;44:3036–55.
Li W, Wang J, Zhang J, Wang W. Molecular simulations of metal-coupled protein folding. Curr Opin Struct Biol. 2015;30:25–31.
Pastoriza-Gallego M, Rabah L, Gibrat G, Thiebot B, van der Goot FG, Auvray L, et al. Dynamics of unfolded protein transport through an aerolysin pore. J Am Chem Soc. 2011;133:2923–31.
Acknowledgements
The authors thank Prof. Yi-Tao Long for providing kind advice on nanopore experiments and data analysis. JW thanks Prof. Mathias Winterhalter's group for kindly providing purified proteins and fruitful discussions and Nanion Technologies GmbH for providing technical support for use of the Orbit 16 system during the experimental work. Y-LY is sponsored by the Chen Guang project supported by Shanghai Municipal Education Commission and Shanghai Education Development Foundation (17CG27).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Published in the topical collection Young Investigators in (Bio-)Analytical Chemistry with guest editors Erin Baker, Kerstin Leopold, Francesco Ricci, and Wei Wang.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 774 kb)
Rights and permissions
About this article
Cite this article
Wang, J., Fertig, N. & Ying, YL. Real-time monitoring β-lactam/β-lactamase inhibitor (BL/BLI) mixture towards the bacteria porin pathway at single molecule level. Anal Bioanal Chem 411, 4831–4837 (2019). https://doi.org/10.1007/s00216-019-01650-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-019-01650-3