Abstract
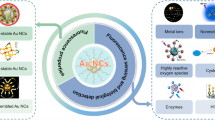
A fluorescent sensing platform using KI3-quenched bovine serum albumin stabilized gold nanoclusters has been designed and used as a fluorescent probe for the turn-on detection of homocysteine/cysteine (Cys/Hcy). The fluorescence of gold nanoclusters was quenched by iodine. The fluorescence of quenched gold nanoclusters was effectively switched on by Cys/Hcy devoid of the interference of glutathione. The transmission electron microscopy image, X-ray photoelectron spectroscopy analysis, time-correlated single photon counting analysis, and dynamic light scattering data confirmed the aggregation-induced quenching of fluorescence of gold nanoclusters by iodine. The turn-on response of Cys/Hcy shows two linear ranges from 0.0057 to 5 μM and from 8 to 25 μM, with a limit of detection of 9 nM for cysteine and 12 nM for homocysteine. Real samples were analyzed to monitor Cys/Hcy added to human serum. The fluorescence turn-on response of the probe on a paper strip in the presence of Cys/Hcy was studied.

ᅟ










Similar content being viewed by others
References
Brattström L, Wilcken DE. Homocysteine and cardiovascular disease: cause or effect? Am J Clin Nutr. 2000;72(2):315–23. https://doi.org/10.1093/ajcn/72.2.315.
Sonoda M, Shoji T, Kuwamura Y, Okute Y, Naganuma T, Shima H, et al. Plasma homocysteine and cerebral small vessel disease as possible mediators between kidney and cognitive functions in patients with diabetes mellitus. Sci Rep. 2017;7(1):4382. https://doi.org/10.1038/s41598-017-04515-w.
Chung KH, Chiou HY, Chen YH. Associations between serum homocysteine levels and anxiety and depression among children and adolescents in Taiwan. Sci Rep. 2017;7(1):8330. https://doi.org/10.1038/s41598-017-08568-9.
Troen AM. The central nervous system in animal models of hyperhomocysteinemia. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29(7):1140–51. https://doi.org/10.1016/j.pnpbp.2005.06.025.
Yasar A, Gunduz K, Onur E, Calkan M. Serum homocysteine, vitamin B12, folic acid levels and methylenetetrahydrofolate reductase (MTHFR) gene polymorphism in vitiligo. Dis Markers. 2012;33(2):85–9. https://doi.org/10.3233/DMA-2012-0908.
Fratoni V, Brandi ML. B vitamins, homocysteine and bone health. Nutrients. 2015;7(4):2176–92. https://doi.org/10.3390/nu7042176.
Guilliams TG. Homocysteine - a risk factor for vascular diseases: guidelines for the clinical practice. J Am Neutraceutical Assoc. 2004;7(1):11–24.
Joshi MB, Baipadithaya G, Balakrishnan A, Hegde M, Vohra M, Ahamed R, et al. Elevated homocysteine levels in type 2 diabetes induce constitutive neutrophil extracellular traps. Sci Rep. 2016;6:36362. https://doi.org/10.1038/srep36362.
Ganguly P, Alam SF. Role of homocysteine in the development of cardiovascular disease. Nutr J. 2015;14:6. https://doi.org/10.1186/1475-2891-14-6.
Jacobsen DW. Homocysteine and vitamins in cardiovascular disease. Clin Chem. 1998;44(8):1833–43.
Eikelboom JW, Lonn E, Genest J Jr, Hankey G, Yusuf S. Homocyst(e)ine and cardiovascular disease: a critical review of the epidemiologic evidence. Ann Intern Med. 1999;131(5):363–75.
Ubbink JB. Assay methods for the measurement of total homocyst(e)ine in plasma. Semin Thromb Hemost. 2000;26(3):233–41. https://doi.org/10.1055/s-2000-8468.
Yin CX, Xiong KM, Huo FJ, Salamanca JC, Strongin RM. Fluorescent probes with multiple binding sites for the discrimination of Cys, Hcy, and GSH. Angew Chem Int Ed Engl. 2017;56(43):13188–98. https://doi.org/10.1002/anie.20170408.
Forman HJ, Zhang H, Rinna A. Glutathione: overview of its protective roles, measurement, and biosynthesis. Mol Aspects Med. 2009;30(1-2):1–12. https://doi.org/10.1016/j.mam.2008.08.006.
Liu Y, Yu D, Ding S, Xiao Q, Guo J, Feng G. Rapid and ratiometric fluorescent detection of cysteine with high selectivity and sensitivity by a simple and readily available probe. ACS Appl Mater Interfaces. 2014;6(20):17543–50. https://doi.org/10.1021/am505501d.
Wu Q, Zhou J, Wu Y, Yu C, Hao E, Jiao L. Highly selective colorimetric and fluorescent BODIPY dyes for sensing of cysteine and/or homocysteine. New J Chem. 2016;40(2):1387–95. https://doi.org/10.1039/c5nj02346g.
Yue Y, Huo F, Li X, Wen Y, Yi T, Salamanca J, et al. pH-dependent fluorescent probe that can be tuned for cysteine or homocysteine. Org Lett. 2017;19(1):82–5. https://doi.org/10.1021/acs.orglett.6b03357.
Apyari VV, Arkhipova VV, Isachenko AI, Volkov PA, Dmitrienko SG. Torocheshnikova II. Label-free gold nanoparticle-based sensing of cysteine: new peculiarities and prospects. Sens Actuators B. 2018;260:953–61. https://doi.org/10.1016/j.snb.2018.01.118.
Sun J, Yang F, Zhao D, Chen C, Yang X. Integrated logic gate for fluorescence turn-on detection of histidine and cysteine based on Ag/Au bimetallic nanoclusters–Cu2+ ensemble. ACS Appl Mater Interfaces. 2015;7(12):6860–6. https://doi.org/10.1021/acsami.5b00434.
Rusin O, St. Luce NN, Agbaria RA, Escobedo JO, Jiang S, Warner IM, et al. Visual detection of cysteine and homocysteine. J Am Chem Soc. 2004;126(2):438–9. https://doi.org/10.1021/ja036297t.
Yue Y, Huo F, Ning P, Zhang Y, Chao J, Meng X, et al. Dual-site fluorescent probe for visualizing the metabolism of Cys in living cells. J Am Chem Soc. 2017;139(8):3181–5. https://doi.org/10.1021/jacs.6b12845.
Liu T, Huo F, Li J, Chao J, Zhang Y, Yin C. An off-on fluorescent probe for specifically detecting cysteine and its application in bioimaging. Sens Actuators B. 2016;237:127–32. https://doi.org/10.1016/j.snb.2016.06.080.
Yue Y, Yin C, Huo F, Chao J, Zhang Y. Thiol-chromene click chemistry: a turn-on fluorescent probe for specific detection of cysteine and its application in bioimaging. Sens Actuators B. 2016;223:496–500. https://doi.org/10.1016/j.snb.2015.09.127.
Liu T, Huo F, Yin C, Li J, Niu L. A highly selective fluorescence sensor for cysteine/homocysteine and its application in bioimaging. RSC Adv. 2015;5(36):28713–6. https://doi.org/10.1039/c5ra03011k.
Li J, Yin C, Zhang Y, Chao J, Huo F. A long wavelength fluorescent probe for biothiols and its application in cell imaging. Anal Methods. 2016;8(37):6748–53. https://doi.org/10.1039/c6ay02150f.
Su Y, Qi L, Mu X, Wang M. A fluorescent probe for sensing ferric ions in bean sprouts based on l-histidine-stabilized gold nanoclusters. Anal Methods. 2015;7(2):684–9. https://doi.org/10.1039/c4ay02186j.
Xie J, Zheng Y, Ying JY. Protein-directed synthesis of highly fluorescent gold nanoclusters. J Am Chem Soc. 2009;131(3):888–9. https://doi.org/10.1021/ja806804u.
Liu H, Li M, Xia Y, Ren X. A turn-on fluorescent sensor for selective and sensitive detection of alkaline phosphatase activity with gold nanoclusters based on inner filter effect. ACS Appl Mater Interfaces. 2017;9(1):120–6. https://doi.org/10.1021/acsami.6b11920.
Chang H-C, Ho J-aA. Gold nanocluster-assisted fluorescent detection for hydrogen peroxide and cholesterol based on the inner filter effect of gold nanoparticles. Anal Chem. 2015;87(20):10362–7. https://doi.org/10.1021/acs.analchem.5b02452.
Wu Y-T, Shanmugam C, Tseng W-B, Hiseh M-M, Tseng W-L. A gold nanocluster-based fluorescent probe for simultaneous pH and temperature sensing and its application to cellular imaging and logic gates. Nanoscale. 2016;8(21):11210–6. https://doi.org/10.1039/c6nr02341j.
Nebu J, Anjali Devi JS, Aparna RS, Abha K, Sony G. Erlotinib conjugated gold nanocluster enveloped magnetic iron oxide nanoparticles–a targeted probe for imaging pancreatic cancer cells. Sens Actuators B. 2018;257:1035–43. https://doi.org/10.1016/j.snb.2017.11.017.
Li R, Xu P, Tu Y, Yan J. Albumin-stabilized gold nanoclusters as viable fluorescent probes in non-titrimetric iodometry for the detection of oxidizing analytes. Microchim Acta. 2016;183(1):497–502. https://doi.org/10.1007/s00604-015-1661-y.
Zhou M, Zeng C, Chen Y, Zhao S, Sfeir MY, Zhu M, et al. Evolution from the plasmon to exciton state in ligand-protected atomically precise gold nanoparticles. Nat Commun. 2016;7:13240. https://doi.org/10.1038/ncomms13240.
Wu Z, Jin R. On the ligand’s role in the fluorescence of gold nanoclusters. Nano Lett. 2010;10(7):2568–73. https://doi.org/10.1021/nl101225f.
Lystvet SM, Volden S, Singh G, Yasuda M, Halskau O, Glomm WR. Tunable photophysical properties, conformation and function of nanosized protein-gold constructs. RSC Adv. 2013;3(2):482–95. https://doi.org/10.1039/c2ra22479h.
Wang H, Xu C, Zheng C, Xu W, Dong T, Liu K, et al. Facile synthesis and characterization of Au nanoclusters-silica fluorescent composite nanospheres. J Nanomater. 2013;2013:5. https://doi.org/10.1155/2013/972834.
Meng H, Yang D, Tu Y, Yan J. Turn-on fluorescence detection of ascorbic acid with gold nanolcusters. Talanta. 2017;165(Suppl C):346–50. https://doi.org/10.1016/j.talanta.2016.12.047.
Jung HS, Han JH, Pradhan T, Kim S, Lee SW, Sessler JL, et al. A cysteine-selective fluorescent probe for the cellular detection of cysteine. Biomaterials. 2012;33(3):945–53. https://doi.org/10.1016/j.biomaterials.2011.10.040.
Yahia-Ammar A, Sierra D, Mérola F, Hildebrandt N, Le Guével X. Self-assembled gold nanoclusters for bright fluorescence imaging and enhanced drug delivery. ACS Nano. 2016;10(2):2591–9. https://doi.org/10.1021/acsnano.5b07596.
Wei YJ, Liu CG, Mo LP. Ultraviolet absorption spectra of iodine, iodide ion and triiodide ion. Spectrosc Spectr Anal. 2005;25(1):86–8.
Li R, Xu P, Fan J, Di J, Tu Y, Yan J. Sensitive iodate sensor based on fluorescence quenching of gold nanocluster. Anal Chim Acta. 2014;827(Suppl C):80–5. https://doi.org/10.1016/j.aca.2014.04.013.
Chen YM, Cheng TL, Tseng WL. Fluorescence turn-on detection of iodide, iodate and total iodine using fluorescein-5-isothiocyanate-modified gold nanoparticles. Analyst. 2009;134(10):2106–12. https://doi.org/10.1039/b905426j.
Huang J, Dai W-L, Fan K. Remarkable support crystal phase effect in Au/FeOx catalyzed oxidation of 1,4-butanediol to γ-butyrolactone. J Catal. 2009;266(2):228–35. https://doi.org/10.1016/j.jcat.2009.06.011.
Klyushin AY, Rocha TCR, Havecker M, Knop-Gericke A, Schlogl R. A near ambient pressure XPS study of Au oxidation. Phys Chem Chem Phys. 2014;16(17):7881–6. https://doi.org/10.1039/c4cp00308j.
Acknowledgements
The authors thank the head of the Department of Chemistry, University of Kerala, Kariavattom campus, Thiruvananthapuram, for providing the platform to conduct the research. The authors also thank the director of the SICC, University of Kerala, Kariavattom campus, Thiruvananthapuram, the director of SAIF-STIC-CUSAT, Kochi, RGCB, Thiruvananthapuram, and DST-SAIF, M.G. University, Kottayam. N.J. acknowledges support for this work by the University Grants Commission, Bangalore, India, through a teacher fellowship (F.No.FIP/12th plan/KLMG035, TF: 03) under the faculty development program during the 12th plan period.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All biological experiments were performed with the approval of the Human Ethics Committee, University of Kerala, Kerala. Informed consent was obtained from all individual participants included in the study
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(PDF 616 kb)
Rights and permissions
About this article
Cite this article
Nebu, J., Anjali Devi, J.S., Aparna, R.S. et al. Potassium triiodide-quenched gold nanocluster as a fluorescent turn-on probe for sensing cysteine/homocysteine in human serum. Anal Bioanal Chem 411, 997–1007 (2019). https://doi.org/10.1007/s00216-018-1511-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1511-y