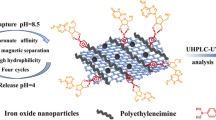
Abstract
In this paper, a high-affinity graphene oxide-encapsulated magnetic Zr-MOF (GO-Mag@Zr-MOF) was synthesized and characterized by SEM, TEM, and XPS for its morphology, structure, and components. Subsequently, the as-prepared GO-Mag@Zr-MOF was, for the first time, employed as magnetic solid-phase extraction (MSPE) adsorbent for pretreatment and determination of photodynamic therapy (PDT) with the photosensitizers hematoporphyrin (Hp) and hematoporphyrin monomethyl ether (HMME) in human urine samples coupled with ultra-performance liquid chromatography-high resolution mass spectrometry (UPLC-HRMS). The synthesized GO-Mag@Zr-MOF revealed excellent adsorption efficiency for Hp and HMME in urine samples. Under optimal conditions, the spiked recoveries of the developed method were in the range of 89.5–105.6% with RSDs less than 10%. The limits of detection (LODs) were found to be 0.036 and 0.042 μg/L for Hp and HMME, respectively, while limits of quantitation (LOQs) were 0.12 and 0.14 μg/L. The proposed method was found to be rapid, effective, sensitive, and accurate for clinical analysis. Moreover, this paper, for the first time, carefully expounded the mass spectrum cracking mechanisms of Hp and HMME.

ᅟ













Similar content being viewed by others
References
Brozek-Pluska B, Kopec M. Raman microspectroscopy of hematoporphyrins. Imaging of the noncancerous and the cancerous human breast tissues with photosensitizers. Spectrochim Acta A Mol Biomol Spectrosc. 2016;169:182–91.
Tian ZD, Quan XM, Xu CS, Dan L, Guo H, Leung WN. Hematoporphyrin monomethyl ether enhances the killing action of ultrasound on osteosarcoma in vivo. J Ultrasound Med. 2009;28:1695–702.
Sibille A, Lambert R, Souquet JC, Sabben G, Descos F. Long-term survival after photodynamic therapy for esophageal cancer. Gastroenterology. 1995;108(2):337–44.
Yang J, Shen H, Jin H, Lou Q, Zhang X. Treatment of unresectable extrahepatic cholangiocarcinoma using hematoporphyrin photodynamic therapy: a prospective study. Photodiagn Photodyn Ther. 2016;16:110–8.
Tang Y, Xie H, Li J, Jian D. The association between treatment reactions and treatment efficiency of Hemoporfin-photodynamic therapy on port wine stains: a prospective double blind randomized controlled trial. Photodiagn Photodyn Ther. 2017;18:171–8.
Wang Y, Zuo Z, Liao X, Gu Y, Qiu H, Zeng J. Investigation of photodynamic therapy optimization for port wine stain using modulation of photosensitizer administration methods. Exp Biol Med. 2013;238(12):1344–9.
Qin ZP, Li KL, Ren L, Liu XJ. Photodynamic therapy of port wine stains-a report of 238 cases. Photodiagn Photodyn Ther. 2007;4(1):53–9.
Qiu H, Gu Y, Wang Y, Huang N. Twenty years of clinical experience with a new modality of vascular-targeted photodynamic therapy for port wine stains. Dermatol Surg. 2011;37(11):1603–10.
Bertoloni G, Lauro FM, Cortella G, Merchat M. Photosensitizing activity of hematoporphyrin on staphylococcus aureus cells. BBA-Gen Subjects. 2000;1475(2):169–74.
Aharon D, Weitman H, Ehrenberg B. The effect of liposomes’ surface electric potential on the uptake of hematoporphyrin. Biochim Biophys Acta. 2011;1808(8):2031–5.
McCaughan JS, Mertens BF, Cho C, Barabash RD, Payton HW. Photodynamic therapy to treat tumors of the extrahepatic biliary ducts. A case report. Arch Surg. 1991;126(1):111–3.
Zhao Y, Tu P, Zhou G, Zhou Z, Lin X, Yang H, et al. Hemoporfin photodynamic therapy for port-wine stain: a randomized controlled trial. PLoS One. 2016;11(5):e0156219.
Klein A, Baumler W, Landthaler M, Babilas P. Laser and IPL treatment of port-wine stains: therapy options, limitations, and practical aspects. Lasers Med Sci. 2011;26(6):845–59.
Gasco MR, Trotta M. Determination of hematoporphyrin and protoporphyrin by ion-pair extraction with chlorpromazine. J Pharm Sci. 1981;70(11):1294–5.
Chan EN, Goodall DM. Capillary electrophoresis analysis of polyhaematoporphyrin, a photosensitizer used in photodynamic therapy. J Chromatogr A. 1993;636(1):171–8.
Houle JM, Strong A. Clinical pharmacokinetics of verteporfin. J Clin Pharmacol. 2002;42(5):547–57.
Chen Z, Song T, Chen X, Wang S, Chen J. Study on the interaction between hematoporphyrin monomethyl ether and DNA and the determination of hematoporphyrin monomethyl ether using the resonance light scattering technique. Spectrochim Acta A Mol Biomol Spectrosc. 2010;77(3):605–11.
Ackroyd R, Brown N, Vernon D, Roberts D, Stephenson T, Marcus S, et al. 5-Aminolevulinic acid photosensitization of dysplastic Barrett's esophagus: a pharmacokinetic study. Photochem Photobiol. 1999;70(4):656–62.
Li P, Sun JG, Huang CR, Pan GY, Xu MJ, Li J, et al. Development and validation of a sensitive quantification method for hematoporphyrin monomethyl ether in plasma using high-performance liquid chromatography with fluorescence detection. Biomed Chromatogr. 2006;20(12):1277–82.
Koster RA, Alffenaar J-WC, Greijdanus B, VanDerNagel JE, Uges DR. Application of sweat patch screening for 16 drugs and metabolites using a fast and highly selective LC-MS/MS method. Ther Drug Monit. 2014;36(1):35–45.
Hoff RB, Rübensam G, Jank L, Barreto F, Peralba MCR, Pizzolato TM, et al. Analytical quality assurance in veterinary drug residue analysis methods: matrix effects determination and monitoring for sulfonamides analysis. Talanta. 2015;132:443–50.
Montesano C, Simeoni MC, Curini R, Sergi M, Sterzo CL, Compagnone D. Determination of illicit drugs and metabolites in oral fluid by microextraction on packed sorbent coupled with LC-MS/MS. Anal Bioanal Chem. 2015;407(13):3647–58.
Soleimani M, Ahmadi M, Madrakian T, Afkhami A. Magnetic solid phase extraction of rizatriptan in human urine samples prior to its spectrofluorimetric determination. Sensors Actuators B Chem. 2018;254:1225–33.
Cheng G, Liu Y-L, Wang Z-G, Zhang J-L, Sun D-H, Ni J-Z. The GO/rGO–Fe3O4 composites with good water-dispersibility and fast magnetic response for effective immobilization and enrichment of biomolecules. J Mater Chem. 2012;22(41):21998.
Cheng G, Wang ZG, Liu YL, Zhang JL, Sun DH, Ni JZ. A graphene-based multifunctional affinity probe for selective capture and sequential identification of different biomarkers from biosamples. Chem Commun. 2012;48(82):10240–2.
Amiri A, Saadati-Moshtaghin HR, Zonoz FM, Targhoo A. Preparation and characterization of magnetic Wells-Dawson heteropoly acid nanoparticles for magnetic solid-phase extraction of aromatic amines in water samples. J Chromatogr A. 2017;1483:64–70.
Sorribas S, Zornoza B, Serra-Crespo P, Gascon J, Kapteijn F, Téllez C, et al. Synthesis and gas adsorption properties of mesoporous silica-NH2-MIL-53(Al) core–shell spheres. Microporous Mesoporous Mater. 2016;225:116–21.
Wan H, Chen C, Wu Z, Que Y, Feng Y, Wang W, et al. Encapsulation of heteropolyanion-based ionic liquid within the metal–organic framework MIL-100(Fe) for biodiesel production. ChemCatChem. 2015;7(3):441–9.
Qu Q, Gao T, Zheng H, Li X, Liu H, Shen M, et al. Graphene oxides-guided growth of ultrafine Co3O4 nanocrystallites from MOFs as high-performance anode of Li-ion batteries. Carbon. 2015;92:119–25.
Ban Y, Li Z, Li Y, Peng Y, Jin H, Jiao W, et al. Confinement of ionic liquids in nanocages: tailoring the molecular sieving properties of ZIF-8 for membrane-based CO2 capture. Angew Chem Int Ed. 2015;127(51):15703–7.
Ahmed I, Panja T, Khan NA, Sarker M, Yu JS, Jhung SH. Nitrogen-doped porous carbons from ionic liquids@MOF: remarkable adsorbents for both aqueous and nonaqueous media. ACS Appl Mater Interfaces. 2017;9(11):10276–85.
Cheng G, Wang ZG, Denagamage S, Zheng SY. Graphene-templated synthesis of magnetic metal organic framework nanocomposites for selective enrichment of biomolecules. ACS Appl Mater Interfaces. 2016;8(16):10234–42.
He Z, Liu D, Li R, Zhou Z, Wang P. Magnetic solid-phase extraction of sulfonylurea herbicides in environmental water samples by Fe3O4@dioctadecyl dimethyl ammonium chloride@silica magnetic particles. Anal Chim Acta. 2012;747(1):29–35.
Li B, Liu Z, Han C, Wei M, Zhao S. In situ synthesis, characterization, and catalytic performance of tungstophosphoric acid encapsulated into the framework of mesoporous silica pillared clay. J Colloid Interface Sci. 2012;377(1):334–41.
Acknowledgements
We would like to thank the Zhejiang Provincial Natural Science Foundation (LQ19B050001), Ningbo Municipal Natural Science Foundation (No. 2018A610404, No. 2016A610178), Ningbo Municipal Program for Leading and Top-Notch Talents, Zhejiang Provincial Program for Public Welfare of Technology Application Research Plan (No. 2015C31148), and the Opening Foundation of Key Laboratory of Emergency Detection for Public Health of Zhejiang Province for their financial support of this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Informed consents were obtained from three volunteers who donated urine samples. This was not a medical study in any form. Urine samples were used to optimize liquid chromatography-mass spectrometry analysis of Hp and HMME.
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Hua, X., Gao, G. & Pan, S. High-affinity graphene oxide-encapsulated magnetic Zr-MOF for pretreatment and rapid determination of the photosensitizers hematoporphyrin and hematoporphyrin monomethyl ether in human urine prior to UPLC-HRMS. Anal Bioanal Chem 410, 7749–7764 (2018). https://doi.org/10.1007/s00216-018-1391-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1391-1