Abstract
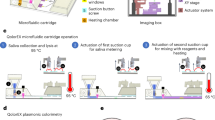
A variety of automated sample-in-answer-out systems for in vitro molecular diagnostics have been presented and even commercialized. Although efficient in operation, they are incapable of quantifying targets, since quantitation based on analog analytical methods (via standard curve analysis) is complex, expensive, and challenging. To address this issue, herein, we describe an integrated sample-in-digital-answer-out (SIDAO) diagnostic system incorporating DNA extraction and digital recombinase polymerase amplification, which enables rapid and quantitative nucleic acid analysis from bodily fluids within a disposable cartridge. Inside the cartridge, reagents are pre-stored in sterilized tubes, with an automated pipetting module allowing facile liquid transfer. For digital analysis, we fabricate a simple, single-layer polydimethylsiloxane microfluidic device and develop a novel and simple sample compartmentalization strategy. Sample solution is partitioned into an array of 40,044 fL-volume microwells by sealing the microfluidic device through the application of mechanical pressure. The entire analysis is performed in a portable, fully automated instrument. We evaluate the quantitative capabilities of the system by analyzing Mycobacterium tuberculosis genomic DNA from both spiked saliva and serum samples, and demonstrate excellent analytical accuracy and specificity. This SIDAO system provides a promising diagnostic platform for quantitative nucleic acid testing at the point-of-care.

ᅟ





Similar content being viewed by others
References
Fauci AS, Morens DM. The perpetual challenge of infectious diseases. N Engl J Med. 2012;366(5):454–61. https://doi.org/10.1056/NEJMra1108296.
Holmes KK, Bertozzi S, Bloom BR, Jha P. Disease control priorities, third edition: volume 6. Major infectious diseases. World Bank Publications; 2017.
Hans R, Marwaha N. Nucleic acid testing-benefits and constraints. Asian J Transfus Sci. 2014;8(1):2–3. https://doi.org/10.4103/0973-6247.126679.
Asmar S, Drancourt M. Rapid culture-based diagnosis of pulmonary tuberculosis in developed and developing countries. Front Microbiol. 2015;6:1184. https://doi.org/10.3389/fmicb.2015.01184.
Lequin RM. Enzyme immunoassay (EIA)/enzyme-linked immunosorbent assay (ELISA). Clin Chem. 2005;51(12):2415–8. https://doi.org/10.1373/clinchem.2005.051532.
Danielson PB, McKiernan HE, Legg KM. Chapter 5—Integrated polymerase chain reaction technologies (sample-to-answer technologies). In: Molecular diagnostics. 3rd ed. Elsevier; 2017. p. 59–78. https://doi.org/10.1016/B978-0-12-802971-8.00005-5.
Perkins MD, Kessel M. What Ebola tells us about outbreak diagnostic readiness. Nat Biotechnol. 2015;33(5):464–9. https://doi.org/10.1038/nbt.3215.
Vynck M, Vandesompele J, Thas O. Quality control of digital PCR assays and platforms. Anal Bioanal Chem. 2017;409(25):5919–31. https://doi.org/10.1007/s00216-017-0538-9.
Duewer DL, Kline MC, Romsos EL, Toman B. Evaluating droplet digital PCR for the quantification of human genomic DNA: converting copies per nanoliter to nanograms nuclear DNA per microliter. Anal Bioanal Chem. 2018;410(12):2879–87. https://doi.org/10.1007/s00216-018-0982-1.
Bruner KM, Murray AJ, Pollack RA, Soliman MG, Laskey SB, Capoferri AA, et al. Defective proviruses rapidly accumulate during acute HIV-1 infection. Nat Med. 2016;22(9):1043–9. https://doi.org/10.1038/nm.4156.
Pavsic J, Devonshire A, Blejec A, Foy CA, Van Heuverswyn F, Jones GM, et al. Inter-laboratory assessment of different digital PCR platforms for quantification of human cytomegalovirus DNA. Anal Bioanal Chem. 2017;409(10):2601–14. https://doi.org/10.1007/s00216-017-0206-0.
Zou Z, Qi P, Qing ZH, Zheng J, Yang S, Chen WJ, et al. Technologies for analysis of circulating tumour DNA: progress and promise. TrAC Trends Anal Chem. 2017;97:36–49. https://doi.org/10.1016/j.trac.2017.08.004.
Gorgannezhad L, Umer M, Islam MN, Nguyen NT, Shiddiky MJA. Circulating tumor DNA and liquid biopsy: opportunities, challenges, and recent advances in detection technologies. Lab Chip. 2018;18(8):1174–96. https://doi.org/10.1039/c8lc00100f.
Ye W, Tang XJ, Liu C, Wen CW, Li W, Lyu JX. Accurate quantitation of circulating cell-free mitochondrial DNA in plasma by droplet digital PCR. Anal Bioanal Chem. 2017;409(10):2727–35. https://doi.org/10.1007/s00216-017-0217-x.
Kakiuchi M, Nishizawa T, Ueda H, Gotoh K, Tanaka A, Hayashi A, et al. Recurrent gain-of-function mutations of RHOA in diffuse-type gastric carcinoma. Nat Genet. 2014;46(6):583–7. https://doi.org/10.1038/ng.2984.
Whale AS, Devonshire AS, Karlin-Neumann G, Regan J, Javier L, Cowen S, et al. International interlaboratory digital PCR study demonstrating high reproducibility for the measurement of a rare sequence variant. Anal Chem. 2017;89(3):1724–33. https://doi.org/10.1021/acs.analchem.6b03980.
Wu Z, Bai Y, Cheng Z, Liu F, Wang P, Yang D, et al. Absolute quantification of DNA methylation using microfluidic chip-based digital PCR. Biosens Bioelectron. 2017;96:339–44. https://doi.org/10.1016/j.bios.2017.05.021.
Pharo HD, Andresen K, Berg KCG, Lothe RA, Jeanmougin M, Lind GE. A robust internal control for high-precision DNA methylation analyses by droplet digital PCR. Clin Epigenetics. 2018;10 https://doi.org/10.1186/s13148-018-0456-5.
Murtaza M, Dawson SJ, Tsui DW, Gale D, Forshew T, Piskorz AM, et al. Non-invasive analysis of acquired resistance to cancer therapy by sequencing of plasma DNA. Nature. 2013;497(7447):108–12. https://doi.org/10.1038/nature12065.
Hata AN, Niederst MJ, Archibald HL, Gomez-Caraballo M, Siddiqui FM, Mulvey HE, et al. Tumor cells can follow distinct evolutionary paths to become resistant to epidermal growth factor receptor inhibition. Nat Med. 2016;22(3):262–9. https://doi.org/10.1038/nm.4040.
Tadmor AD, Ottesen EA, Leadbetter JR, Phillips R. Probing individual environmental bacteria for viruses by using microfluidic digital PCR. Science. 2011;333(6038):58–62. https://doi.org/10.1126/science.1200758.
Mehle N, Dobnik D, Ravnikar M, Novak MP. Validated reverse transcription droplet digital PCR serves as a higher order method for absolute quantification of potato virus Y strains. Anal Bioanal Chem. 2018;410(16):3815–25. https://doi.org/10.1007/s00216-018-1053-3.
Niu CQ, Xu YC, Zhang C, Zhu PY, Huang KL, Luo YB, et al. Ultrasensitive single fluorescence-labeled probe-mediated single universal primer-multiplex-droplet digital polymerase chain reaction for high-throughput genetically modified organism screening. Anal Chem. 2018;90(9):5586–93. https://doi.org/10.1021/acs.analchem.7b03974.
Demeke T, Dobnik D. Critical assessment of digital PCR for the detection and quantification of genetically modified organisms. Anal Bioanal Chem. 2018;410(17):4039–50. https://doi.org/10.1007/s00216-018-1010-1.
Piepenburg O, Williams CH, Stemple DL, Armes NA. DNA detection using recombination proteins. PLoS Biol. 2006;4(7):1115–21. https://doi.org/10.1371/journal.pbio.0040204.
Stringer OW, Andrews JM, Greetham HL, Forrest MS. TwistAmp (R) Liquid: a versatile amplification method to replace PCR. Nat Methods. 2018;15(5):I–Iii.
Yeh EC, Fu CC, Hu L, Thakur R, Feng J, Lee LP. Self-powered integrated microfluidic point-of-care low-cost enabling (SIMPLE) chip. Sci Adv. 2017;3(3) https://doi.org/10.1126/sciadv.1501645.
Schuler F, Schwemmer F, Trotter M, Wadle S, Zengerle R, von Stetten F, et al. Centrifugal step emulsification applied for absolute quantification of nucleic acids by digital droplet RPA. Lab Chip. 2015;15(13):2759–66. https://doi.org/10.1039/c5lc00291e.
Li Z, Liu Y, Wei QQ, Liu YJ, Liu WW, Zhang XL, et al. Picoliter well array chip-based digital recombinase polymerase amplification for absolute quantification of nucleic acids. PLoS One. 2016;11(4) https://doi.org/10.1371/journal.pone.0153359.
Ven KR, Vanspauwen B, Perez-Ruiz E, Leirs K, Decrop D, Gerstmans H, et al. Target confinement in small reaction volumes using microfluidic technologies: a smart approach for single-entity detection and analysis. ACS Sensors. 2018;3(2):264–84. https://doi.org/10.1021/acssensors.7b00873.
Cao L, Cui XY, Hu J, Li ZD, Choi JR, Yang QZ, et al. Advances in digital polymerase chain reaction (dPCR) and its emerging biomedical applications. Biosens Bioelectron. 2017;90:459–74. https://doi.org/10.1016/j.bios.2016.09.082.
Zhang YH, Jiang HR. A review on continuous-flow microfluidic PCR in droplets: advances, challenges and future. Anal Chim Acta. 2016;914:7–16. https://doi.org/10.1016/j.aca.2016.02.006.
Zhu PA, Wang LQ. Passive and active droplet generation with microfluidics: a review. Lab Chip. 2017;17(1):34–75. https://doi.org/10.1039/c6lc01018k.
Kosir AB, Divieto C, Pavsic J, Pavarelli S, Dobnik D, Dreo T, et al. Droplet volume variability as a critical factor for accuracy of absolute quantification using droplet digital PCR. Anal Bioanal Chem. 2017;409(28):6689–97. https://doi.org/10.1007/s00216-017-0625-y.
Men YF, Fu YS, Chen ZT, Sims PA, Greenleaf WJ, Huang YY. Digital polymerase chain reaction in an array of femtoliter polydimethylsiloxane microreactors. Anal Chem. 2012;84(10):4262–6. https://doi.org/10.1021/ac300761n.
Heyries KA, Tropini C, VanInsberghe M, Doolin C, Petriv OI, Singhal A, et al. Megapixel digital PCR. Nat Methods. 2011;8(8):649–U64. https://doi.org/10.1038/nmeth.1640.
Low HY, Chan SJ, Soo GH, Ling B, Tan EL. Clarity (TM) digital PCR system: a novel platform for absolute quantification of nucleic acids. Anal Bioanal Chem. 2017;409(7):1869–75. https://doi.org/10.1007/s00216-016-0131-7.
Shen F, Sun B, Kreutz JE, Davydova EK, Du W, Reddy PL, et al. Multiplexed quantification of nucleic acids with large dynamic range using multivolume digital RT-PCR on a rotational SlipChip tested with HIV and hepatitis C viral load. J Am Chem Soc. 2011;133(44):17705–12. https://doi.org/10.1021/ja2060116.
Hindson CM, Chevillet JR, Briggs HA, Gallichotte EN, Ruf IK, Hindson BJ, et al. Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Methods. 2013;10(10):1003–5. https://doi.org/10.1038/nmeth.2633.
Baker M. Digital PCR hits its stride. Nat Methods. 2012;9(6):541–4. https://doi.org/10.1038/nmeth.2027.
Chen H, Wu YQ, Chen Z, Hu ZL, Fang YL, Liao P, et al. Performance evaluation of a novel sample in-answer out (SIAO) system based on magnetic nanoparticles. J Biomed Nanotechnol. 2017;13(12):1619–30. https://doi.org/10.1166/jbn.2017.247.
Ali Z, Liang WB, Jin L, Tang YJ, Mou XB, Shah MAA, et al. Development of magnetic nanoparticles based nucleic acid extraction method and application in hepatitis C virus chemiluminescent detection. Sci Adv Mater. 2015;7(7):1233–40. https://doi.org/10.1166/sam.2015.1974.
Ali ZS, Wang JH, Mou XB, Tang YJ, Li TT, Liang WB, et al. Integration of nucleic acid extraction protocol with automated extractor for multiplex viral detection. J Nanosci Nanotechnol. 2017;17(2):862–70. https://doi.org/10.1166/jnn.2017.12613.
Wang JH, Ali ZS, Wang NY, Liang WB, Liu HN, Li F, et al. Simultaneous extraction of DNA and RNA from Escherichia coli BL 21 based on silica-coated magnetic nanoparticles. Sci China Chem. 2015;58(11):1774–8. https://doi.org/10.1007/s11426-015-5483-x.
Zhu QY, Xu YN, Qiu L, Ma CC, Yu BW, Song Q, et al. A scalable self-priming fractal branching microchannel net chip for digital PCR. Lab Chip. 2017;17(9):1655–65. https://doi.org/10.1039/C7LC00267J.
INTERNATIONAL STANDARD ISO 8655-2 (E), First edition. Piston-operated volumetric apparatus—part 2: piston pipettes. British Standards Institution; 2002.
Acknowledgments
This work was supported by the Swiss Federal Institute of Technology (ETH Zürich), the National Research Foundation of Korea (Grant Nos. 2008-0061891 and 2009-00426), the National Natural Science Foundation of China (Grant No. 61527806), the Hunan Key Research Project (Grant No. 2017SK2174), and the China Postdoctoral Science Foundation (Grant No. 2018M630498). P.D.H. acknowledges support from European Union’s Horizon 2020 research and innovation program through the Individual Marie Skłodowska-Curie Fellowship “Ampidots” under grant agreement no. 701994.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
The studies described herein were approved by Nanjing Drum Tower Hospital Clinical Research Ethics Committee, and have been performed in accordance with ethical standards. Informed consent was obtained from all participants.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
ABC Highlights: authored by Rising Stars and Top Experts.
Electronic supplementary material
ESM 1
(PDF 356 kb)
Rights and permissions
About this article
Cite this article
Yang, H., Chen, Z., Cao, X. et al. A sample-in-digital-answer-out system for rapid detection and quantitation of infectious pathogens in bodily fluids. Anal Bioanal Chem 410, 7019–7030 (2018). https://doi.org/10.1007/s00216-018-1335-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1335-9