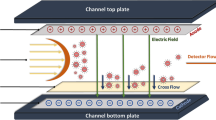
Abstract
Flow field-flow fractionation (FlFFF) with inductively coupled plasma mass spectrometric (ICP-MS) detection was applied for estimating the hydrodynamic diameter of gold nanoparticles (AuNPs). Hydrodynamic diameters of AuNPs of the same core diameter but with different surface coatings were different because the coating agents and their properties were different. The challenge of this work is due to the fact that AuNPs with various types of surface coatings exhibited different interactions in the FlFFF channel, leading to different retention behaviors. Therefore, we are interested in finding suitable FlFFF conditions for estimating the hydrodynamic diameter of AuNPs with various types of electrostatic stabilizing agents [tannic acid (TA) and citrate (CT)] and steric stabilizing agents [polyethylene glycol (PEG), polyvinylpyrrolidone (PVP), and branched polyethylene imine (BPEI)]. Different types of carrier liquids (DI water, 0.02% FL-70, 0.05% SDS, and 30 mM Tris buffer) and membrane materials [regenerated cellulose (RC) and polyethersulfone (PES) membranes] were investigated. Generally, FlFFF was applied for size characterization of nanoparticles based on FlFFF theory but the interactions between AuNPs and membrane affected the retention and the experimentally obtained hydrodynamic diameters of AuNPs from the FlFFF system. With DI water as a carrier liquid with RC or PES membranes, the hydrodynamic diameters of negatively charged particles (TA-, CT-, PVP-, and PEG-stabilized AuNPs) from FlFFF corresponded well with the hydrodynamic diameters from dynamic light scattering (DLS). Interestingly, it was possible to estimate hydrodynamic diameters of AuNPs in the mixture by using FlFFF whereas it was not possible with the use of DLS within the size range studied. This work summarized the possible interactions between AuNPs with various coating agents and membrane materials in different carrier liquids to give guidelines on the suitable conditions of FlFFF for further applications on AuNP hydrodynamic diameter estimation.







Similar content being viewed by others
References
Samontha A, Shiowatana J, Siripinyanond A. Particle size characterization of titanium dioxide in sunscreen products using sedimentation field-flow fractionation–inductively coupled plasma–mass spectrometry. Anal Bioanal Chem. 2011;399:973–8.
Monteiro DR, Gorup LF, Takamiya AS, Ruvollo-Filho AC, Camargo ER, Barbosa DB. The growing importance of materials that prevent microbial adhesion: antimicrobial effect of medical devices containing silver. Int J Antimicrob Agents. 2009;34:103–10.
Hone DC, Walker PI, Evans-Gowing R, FitzGerald S, Beeby A, Chambrier I, et al. Generation of cytotoxic singlet oxygen via phthalocyanine-stabilized gold nanoparticles: a potential delivery vehicle for photodynamic therapy. Langmuir. 2002;18:2985–7.
Kneipp J, Kneipp H, Rice WL, Kneipp K. Optical probes for biological applications based on surface-enhanced raman scattering from indocyanine green on gold nanoparticles. Anal Chem. 2005;77:2381–5.
Hutter E, Maysinger D. Gold nanoparticles and quantum dots for bioimaging. Microsc Res Tech. 2011;74:592–604.
Lai S-F, Ko B-H, Chien C-C, Chang C-J, Yang S-M, Chen H-H, et al. Gold nanoparticles as multimodality imaging agents for brain gliomas. J Nanobiotechnology. 2015;13:85.
Clement S, Chen W, Anwer AG, Goldys EM. Verteprofin conjugated to gold nanoparticles for fluorescent cellular bioimaging and X-ray mediated photodynamic therapy. Microchim Acta. 2017;184:1765–71.
Schimpf ME, Caldwell JC, Giddings JC (eds). Field-flow fractionation handbook. New York: Wiley; 2000.
Giddings JC, Yang FJ, Myers MN. Flow-field-flow fractionation: a versatile new separation method. Science. 1976;193:1244.
Calzolai L, Gilliland D, Garcìa CP, Rossi F. Separation and characterization of gold nanoparticle mixtures by flow-field-flow fractionation. J Chromatogr A. 2011;1218:4234–9.
Meisterjahn B, Wagner S, von der Kammer F, Hennecke D, Hofmann T. Silver and gold nanoparticle separation using asymmetrical flow-field flow fractionation: influence of run conditions and of particle and membrane charges. J Chromatogr A. 2016;1440:150–9.
Mudalige TK, Qu H, Sánchez-Pomales G, Sisco PN, Linder SW. Simple functionalization strategies for enhancing nanoparticle separation and recovery with asymmetric flow field flow fractionation. Anal Chem. 2015;87:1764–72.
Bolea E, Jiménez-Lamana J, Laborda F, Castillo JR. Size characterization and quantification of silver nanoparticles by asymmetric flow field-flow fractionation coupled with inductively coupled plasma mass spectrometry. Anal Bioanal Chem. 2011;401:2723–32.
Bendixen N, Losert S, Adlhart C, Lattuada M, Ulrich A. Membrane–particle interactions in an asymmetric flow field flow fractionation channel studied with titanium dioxide nanoparticles. J Chromatogr A. 2014;1334:92–100.
López-Heras I, Madrid Y, Cámara C. Prospects and difficulties in TiO2 nanoparticles analysis in cosmetic and food products using asymmetrical flow field-flow fractionation hyphenated to inductively coupled plasma mass spectrometry. Talanta. 2014;124:71–8.
M-M P, Somchue W, Shiowatana J, Siripinyanond A. Flow field-flow fractionation for particle size characterization of selenium nanoparticles incubated in gastrointestinal conditions. Food Res Int. 2014;57:203–9.
Heroult J, Nischwitz V, Bartczak D, Goenaga-Infante H. The potential of asymmetric flow field-flow fractionation hyphenated to multiple detectors for the quantification and size estimation of silica nanoparticles in a food matrix. Anal Bioanal Chem. 2014;406:3919–27.
Ulrich A, Losert S, Bendixen N, Al-Kattan A, Hagendorfer H, Nowack B, et al. Critical aspects of sample handling for direct nanoparticle analysis and analytical challenges using asymmetric field flow fractionation in a multi-detector approach. J Anal At Spectrom. 2012;27:1120–30.
Hagendorfer H, Kaegi R, Traber J, Mertens SFL, Scherrers R, Ludwig C, et al. Application of an asymmetric flow field flow fractionation multi-detector approach for metallic engineered nanoparticle characterization—prospects and limitations demonstrated on Au nanoparticles. Anal Chim Acta. 2011;706:367–78.
Saenmuangchin R, Mettakoonpitak J, Shiowatana J, Siripinyanond A. Separation of silver nanoparticles by hollow fiber flow field-flow fractionation: addition of tannic acid into carrier liquid as a modifier. J Chromatogr A. 2015;1415:115–22.
Jochem A-R, Ankah GN, Meyer L-A, Elsenberg S, Johann C, Kraus T. Colloidal mechanisms of gold nanoparticle loss in asymmetric flow field-flow fractionation. Anal Chem. 2016;88:10065–73.
Gray EP, Bruton TA, Higgins CP, Halden RU, Westerhoff P, Ranville JF. Analysis of gold nanoparticle mixtures: a comparison of hydrodynamic chromatography (HDC) and asymmetrical flow field-flow fractionation (AF4) coupled to ICP-MS. J Anal At Spectrom. 2012;27:1532–9.
Poda AR, Bednar AJ, Kennedy AJ, Harmon A, Hull M, Mitrano DM, et al. Characterization of silver nanoparticles using flow-field flow fractionation interfaced to inductively coupled plasma mass spectrometry. J Chromatogr A. 2011;1218:4219–25.
Geiss O, Cascio C, Gilliland D, Franchini F, Barrero-Moreno J. Size and mass determination of silver nanoparticles in an aqueous matrix using asymmetric flow field flow fractionation coupled to inductively coupled plasma mass spectrometer and ultraviolet–visible detectors. J Chromatogr A. 2013;1321:100–8.
Sánchez-García L, Bolea E, Laborda F, Cubel C, Ferrer P, Gianolio D, et al. Size determination and quantification of engineered cerium oxide nanoparticles by flow field-flow fractionation coupled to inductively coupled plasma mass spectrometry. J Chromatogr A. 2016;1438:205–15.
Qu H, Quevedo IR, Linder SW, Fong A, Mudalige TK. Importance of material matching in the calibration of asymmetric flow field-flow fractionation: material specificity and nanoparticle surface coating effects on retention time. J Nanopart Res. 2016;18:292.
Gigault J, Mignard E, Hadri HE, Grassl B. Measurement bias on nanoparticle size characterization by asymmetric flow field-flow fractionation using dynamic light-scattering detection. Chromatographia. 2017;80:287–94.
Karl M Krueger AMA-S, Mejia M and Colvin VL. The hydrodynamic size of polymer stabilized nanocrystals. Nanotechnology. 2007; 18.
Hackley VA, Clogston JD. Measuring the hydrodynamic size of nanoparticles in aqueous media using batch-mode dynamic light scattering. In: McNeil SE, editor. Characterization of nanoparticles intended for drug delivery. New York: Humana; 2011.
JitKang L, Swee PY, Hui XC, Siew CL. Characterization of magnetic nanoparticle by dynamic light scattering. Nanoscale Res Lett 2013; 8.
Tejamaya M, Römer I, Merrifield RC, Lead JR. Stability of citrate, PVP, and PEG coated silver nanoparticles in ecotoxicology media. Environ Sci Technol. 2012;46:7011–7.
Liu S, Zeng J, Tao D, Zhang L. Microfiltration performance of regenerated cellulose membrane prepared at low temperature for wastewater treatment. Cellulose. 2010;17:1159–69.
Hongo T, Yamane C, Saito M, Okajima K. Super-molecular structures controlling the swelling behavior of regenerated cellulose membranes. Polym J. 1996;28:769.
Farsi M, Heydarinasab A, Honarvar B, Arjmand M. The effect of crosslink temperature upon density and swelling degree of PDMS, PEG, PES and PAN membranes. Research article 6, 2016.
Izák P, Hovorka Š, Bartovský T, Bartovská L, Crespo JG. Swelling of polymeric membranes in room temperature ionic liquids. J Membr Sci. 2007;296:131–8.
Wang L, Yi BL, Zhang HM, Xing DM. Characteristics of polyethersulfone/sulfonated polyimide blend membrane for proton exchange membrane fuel cell. J Phys Chem B. 2008;112:4270–5.
Miller JC, Miller JN. Statistics for analytical chemistry. England: Eillis Horwood Limited; 1993.
Majewska-Nowak K, Kowalska I, Kabsch-Korbutowicz M. Ultrafiltration of SDS solutions using polymeric membranes. Desalination. 2005;184:415–22.
Gigault J, Pettibone JM, Schmitt C, Hackley VA. Rational strategy for characterization of nanoscale particles by asymmetric-flow field flow fractionation: a tutorial. Anal Chim Acta. 2014;809:9–24.
Mudalige TK, Qu H, Linder SW. Rejection of commonly used electrolytes in asymmetric flow field flow fractionation: effects of membrane molecular weight cutoff size, fluid dynamics, and valence of electrolytes. Langmuir. 2017;33:1442–50.
Hassan PA, Rana S, Verma G. Making sense of Brownian motion: colloid characterization by dynamic light scattering. Langmuir. 2015;31:3–12.
Acknowledgements
We are thankful for the research grant (BRG 6180006) from the Thailand Research Fund (TRF) and Mahidol University given to A. Siripinyanond and the scholarship from TRF through the Research and Researchers for Industries (RRi) and Natural Fruit Company Limited (Grant no. PHD 58I0077) given to R. Seanmuangchin. Thanks are also due to the Center of Excellence for Innovation in Chemistry: Postgraduate Education and Research Program in Chemistry (PERCH-CIC), Office of the Higher Education Commission, Ministry of Education, Thailand, and Mahidol University under the National Research Universities Initiative for the support of chemicals and equipment. We are also grateful to the National Nanotechnology Center (NANOTEC) for allowing us to use the zetasizer.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interests.
Electronic supplementary material
ESM 1
(PDF 872 kb)
Rights and permissions
About this article
Cite this article
Saenmuangchin, R., Siripinyanond, A. Flow field-flow fractionation for hydrodynamic diameter estimation of gold nanoparticles with various types of surface coatings. Anal Bioanal Chem 410, 6845–6859 (2018). https://doi.org/10.1007/s00216-018-1284-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1284-3