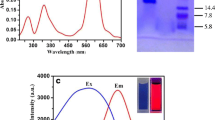
Abstract
The study suggests an application of a coelenteramide-containing fluorescent protein (CLM-CFP) as a simplest bioassay for gamma radiation exposures. “Discharged obelin,” a product of the bioluminescence reaction of the marine coelenterate Obelia longissima, was used as a representative of the CLM-CFP group. The bioassay is based on a simple enzymatic reaction—photochemical proton transfer in the coelenteramide-apoprotein complex. Components of this reaction differ in fluorescence color, providing, by this, an evaluation of the proton transfer efficiency in the photochemical process. This efficiency depends on the microenvironment of the coelenteramide within the protein complex, and, hence, can evaluate a destructive ability of gamma radiation. The CLM-CFP samples were exposed to gamma radiation (137Cs, 2 mGy/h) for 7 and 16 days at 20 °C and 5 °C, respectively. As a result, two fluorescence characteristics (overall fluorescence intensity and contributions of color components to the fluorescence spectra) were identified as bioassay parameters. Both parameters demonstrated high sensitivity of the CLM-CFP-based bioassay to the low-dose gamma radiation exposure (up to 100 mGy). Higher temperature (20 °C) enhanced the response of CLM-CFP to gamma radiation. This new bioassay can provide fluorescent multicolor assessment of protein destruction in cells and physiological liquids under exposure to low doses of gamma radiation.

ᅟ






Similar content being viewed by others

References
Ilyin LA, Kutsenko SA, Savateev NV, Sofronov GA, Tiunov LA. Toxicological problems in mitigation strategies of chemical industries. 1990;35:440–7.
Bisswanger H. Enzyme assays. Report Enzymol Data – STRENDA Recomm Beyond. 2014;1:41–55. https://doi.org/10.1016/j.pisc.2014.02.005.
Acker MG, Auld DS. Considerations for the design and reporting of enzyme assays in high-throughput screening applications. Report Enzymol Data – STRENDA Recomm Beyond. 2014;1:56–73. https://doi.org/10.1016/j.pisc.2013.12.001.
Kratasyuk VA, Esimbekova EN. Applications of luminous bacteria enzymes in toxicology. Comb Chem High Throughput Screen. 2015;18:952–9. https://doi.org/10.2174/1386207318666150917100257.
Zhikrevetskaya S, Peregudova D, Danilov A, Plyusnina E, Krasnov G, Dmitriev A, et al. Effect of low doses (5-40 cGy) of gamma-irradiation on lifespan and stress-related genes expression profile in Drosophila melanogaster. PLoS One. 2015;10:e0133840. https://doi.org/10.1371/journal.pone.0133840.
Bolsunovsky A, Frolova T, Dementyev D, Sinitsyna O. Low doses of gamma-radiation induce SOS response and increase mutation frequency in Escherichia coli and Salmonella typhimurium cells. Ecotoxicol Environ Saf. 2016;134:233–8. https://doi.org/10.1016/j.ecoenv.2016.09.009.
Tigini V, Giansanti P, Mangiavillano A, Pannocchia A, Varese GC. Evaluation of toxicity, genotoxicity and environmental risk of simulated textile and tannery wastewaters with a battery of biotests. Ecotoxicol Environ Saf. 2011;74:866–73. https://doi.org/10.1016/j.ecoenv.2010.12.001.
Thakur MS, Ragavan KV. Biosensors in food processing. J Food Sci Technol. 2013;50:625–41. https://doi.org/10.1007/s13197-012-0783-z.
Roda A, Guardigli M, Michelini E, Mirasoli M. Bioluminescence in analytical chemistry and in vivo imaging. TrAC Trends Anal Chem. 2009;28:307–22. https://doi.org/10.1016/j.trac.2008.11.015.
Thomas DJL, Tyrrel SF, Smith R, Farrow S. Bioassays for the evaluation of landfill leachate toxicity. J Toxicol Environ Health Part B. 2009;12:83–105. https://doi.org/10.1080/10937400802545292.
Ivask A, Rõlova T, Kahru A. A suite of recombinant luminescent bacterial strains for the quantification of bioavailable heavy metals and toxicity testing. BMC Biotechnol. 2009;9:41. https://doi.org/10.1186/1472-6750-9-41.
Kudryasheva N, Kratasyuk V, Esimbekova E, Vetrova E, Nemtseva E, Kudinova I. Development of bioluminescent bioindicators for analysis of environmental pollution. Field Anal Chem Technol. 1998;2:277–80. https://doi.org/10.1002/(SICI)1520-6521(1998)2:5<277::AID-FACT4>3.0.CO;2-P.
Ranjan R, Rastogi NK, Thakur MS. Development of immobilized biophotonic beads consisting of Photobacterium leiognathi for the detection of heavy metals and pesticide. J Hazard Mater. 2012;225–226:114–23. https://doi.org/10.1016/j.jhazmat.2012.04.076.
Girotti S, Ferri EN, Fumo MG, Maiolini E. Monitoring of environmental pollutants by bioluminescent bacteria. Anal Chim Acta. 2008;608:2–29. https://doi.org/10.1016/j.aca.2007.12.008.
Efremenko EN, Maslova OV, Kholstov AV, Senko OV, Ismailov AD. Biosensitive element in the form of immobilized luminescent photobacteria for detecting ecotoxicants in aqueous flow-through systems. Luminescence. 2016;31:1283–9. https://doi.org/10.1002/bio.3104.
Roda A, Guardigli M. Analytical chemiluminescence and bioluminescence: latest achievements and new horizons. Anal Bioanal Chem. 2012;402:69–76. https://doi.org/10.1007/s00216-011-5455-8.
Fedorova GF, Menshov VA, Trofimov AV, Tsaplev YB, Vasil’ev RF, Yablonskaya OI. Chemiluminescence of cigarette smoke: salient features of the phenomenon. Photochem Photobiol. 2017;93:579–89. https://doi.org/10.1111/php.12689.
Tarasova AS, Kislan SL, Fedorova ES, Kuznetsov AM, Mogilnaya OA, Stom DI, et al. Bioluminescence as a tool for studying detoxification processes in metal salt solutions involving humic substances. J Photochem Photobiol B. 2012;117:164–70. https://doi.org/10.1016/j.jphotobiol.2012.09.020.
Kudryasheva NS, Tarasova AS. Pollutant toxicity and detoxification by humic substances: mechanisms and quantitative assessment via luminescent biomonitoring. Environ Sci Pollut Res. 2015;22:155–67. https://doi.org/10.1007/s11356-014-3459-6.
Kudryasheva NS, Rozhko TV. Effect of low-dose ionizing radiation on luminous marine bacteria: radiation hormesis and toxicity. J Environ Radioact. 2015;142:68–77. https://doi.org/10.1016/j.jenvrad.2015.01.012.
Min J, Lee CW, Gu MB. Gamma-radiation dose-rate effects on DNA damage and toxicity in bacterial cells. Radiat Environ Biophys. 2003;42:189–92. https://doi.org/10.1007/s00411-003-0205-8.
Ptitsyn LR, Horneck G, Komova O, Kozubek S, Krasavin EA, Bonev M, et al. A biosensor for environmental genotoxin screening based on an SOS lux assay in recombinant Escherichia coli cells. Appl Environ Microbiol. 1997;63:4377–84.
Rozhko TV, Kudryasheva NS, Kuznetsov AM, Vydryakova GA, Bondareva LG, Bolsunovsky AY. Effect of low-level [small alpha]-radiation on bioluminescent assay systems of various complexity. Photochem Photobiol Sci. 2007;6:67–70. https://doi.org/10.1039/B614162P.
Rozhko TV, Badun GA, Razzhivina IA, Guseynov OA, Guseynova VE, Kudryasheva NS. On the mechanism of biological activation by tritium. J Environ Radioact. 2016;157:131–5. https://doi.org/10.1016/j.jenvrad.2016.03.017.
Selivanova MA, Mogilnaya OA, Badun GA, Vydryakova GA, Kuznetsov AM, Kudryasheva NS. Effect of tritium on luminous marine bacteria and enzyme reactions. J Environ Radioact. 2013;120:19–25. https://doi.org/10.1016/j.jenvrad.2013.01.003.
Alexandrova M, Rozhko T, Vydryakova G, Kudryasheva N. Effect of americium-241 on luminous bacteria. Role of peroxides. J Environ Radioact. 2011;102:407–11. https://doi.org/10.1016/j.jenvrad.2011.02.011.
Esimbekova EN, Kondik AM, Kratasyuk VA. Bioluminescent enzymatic rapid assay of water integral toxicity. Environ Monit Assess. 2013;185:5909–16. https://doi.org/10.1007/s10661-012-2994-1.
Kudryasheva NS, Kovel ES, Sachkova AS, Vorobeva AA, Isakova VG, Churilov GN. Bioluminescent enzymatic assay as a tool for studying antioxidant activity and toxicity of bioactive compounds. Photochem Photobiol. 2017; https://doi.org/10.1111/php.12639.
Kudryasheva NS. Bioluminescence and exogenous compounds: physico-chemical basis for bioluminescent assay. J Photochem Photobiol B. 2006;83:77–86. https://doi.org/10.1016/j.jphotobiol.2005.10.003.
Nemtseva EV, Kudryasheva NS. The mechanism of electronic excitation in the bacterial bioluminescent reaction. Russ Chem Rev. 2007;76:91.
Tarasova AS, Stom DI, Kudryasheva NS. Effect of humic substances on toxicity of inorganic oxidizer bioluminescent monitoring. Environ Toxicol Chem. 2011;30:1013–7. https://doi.org/10.1002/etc.472.
Vetrova EV, Kudryasheva NS, Kratasyuk VA. Redox compounds influence on the NAD(P)H:FMN-oxidoreductase-luciferase bioluminescent system. Photochem Photobiol Sci. 2007;6:35–40. https://doi.org/10.1039/B608152E.
Kirillova TN, Gerasimova MA, Nemtseva EV, Kudryasheva NS. Effect of halogenated fluorescent compounds on bioluminescent reactions. Anal Bioanal Chem. 2011;400:343–51. https://doi.org/10.1007/s00216-011-4716-x.
Sachkova AS, Kovel ES, Churilov GN, Guseynov OA, Bondar AA, Dubinina IA, et al. On mechanism of antioxidant effect of fullerenols. Biochem Biophys Rep. 2017;9:1–8. https://doi.org/10.1016/j.bbrep.2016.10.011.
Vetrova EV, Kudryasheva NS, Visser AJWG, van Hoek A. Characteristics of endogenous flavin fluorescence of Photobacterium leiognathi luciferase and Vibrio fischeri NAD(P)H:FMN-oxidoreductase. Luminescence. 2005;20:205–9. https://doi.org/10.1002/bio.815.
Alieva RR, Kudryasheva NS. Variability of fluorescence spectra of coelenteramide-containing proteins as a basis for toxicity monitoring. Talanta. 2017;170:425–31. https://doi.org/10.1016/j.talanta.2017.04.043.
Belogurova NV, Kudryasheva NS. Discharged photoprotein obelin: fluorescence peculiarities. J Photochem Photobiol B. 2010;101:103–8. https://doi.org/10.1016/j.jphotobiol.2010.07.001.
Belogurova NV, Kudryasheva NS, Alieva RR, Sizykh AG. Spectral components of bioluminescence of aequorin and obelin. J Photochem Photobiol B. 2008;92:117–22. https://doi.org/10.1016/j.jphotobiol.2008.05.006.
Alieva RR, Tomilin FN, Kuzubov AA, Ovchinnikov SG, Kudryasheva NS. Ultraviolet fluorescence of coelenteramide and coelenteramide-containing fluorescent proteins. Experimental and theoretical study. J Photochem Photobiol B. 2016;162:318–23. https://doi.org/10.1016/j.jphotobiol.2016.07.004.
Alieva RR, Belogurova NV, Petrova AS, Kudryasheva NS. Effects of alcohols on fluorescence intensity and color of a discharged-obelin-based biomarker. Anal Bioanal Chem. 2014;406:2965–74. https://doi.org/10.1007/s00216-014-7685-z.
Petrova AS, Alieva RR, Belogurova NV, Tirranen LS, Kudryasheva NS. Variation of spectral characteristics of coelenteramide-containing fluorescent protein from Obelia longissima exposed to dimethyl sulfoxide. Russ Phys J. 2016;59:562–7. https://doi.org/10.1007/s11182-016-0806-8.
Alieva RR, Belogurova NV, Petrova AS, Kudryasheva NS. Fluorescence properties of Ca2+−independent discharged obelin and its application prospects. Anal Bioanal Chem. 2013;405:3351–8. https://doi.org/10.1007/s00216-013-6757-9.
Petrova AS, Lukonina AA, Badun GA, Kudryasheva NS. Fluorescent coelenteramide-containing protein as a color bioindicator for low-dose radiation effects. Anal Bioanal Chem. 2017;409:4377–81. https://doi.org/10.1007/s00216-017-0404-9.
Kudryasheva NS, Petrova AS, Dementyev DV, Bondar AA. Exposure of luminous marine bacteria to low-dose gamma radiation. J Environ Radioact. 2017;169-170:64–9. https://doi.org/10.1016/j.jenvrad.2017.01.002.
Rozhko TV, Guseynov OA, Bondar АА, Guseynova VE, Devyatlovskaya AN, Kudryasheva NS. Is bacterial luminescence response to low-dose radiation associated with mutagenicity. J Environ Radioact. 2017;177:261–5. https://doi.org/10.1016/j.jenvrad.2017.07.010.
Illarionov BA, Frank LA, Illarionova VA, Bondar VS, Vysotski ES, Blinks JR. Recombinant obelin: cloning and expression of cDNA, purification, and characterization as a calcium indicator. Methods Enzymol Academic Press. 2000;305:223–49.
Bolsunovsky AY, Tcherkezian VO. Hot particles of the Yenisei River flood plain, Russia. J Environ Radioact. 2001;57:167–74.
Bolsunovsky A, Melgunov M, Chuguevskii A, Lind OC, Salbu B. Unique diversity of radioactive particles found in the Yenisei River floodplain. Sci Rep. 2017;7:1–10. https://doi.org/10.1038/s41598-017-11557-7.
Shimomura O, Teranishi K. Light-emitters involved in the luminescence of coelenterazine. Luminescence. 2000;15:51–8. https://doi.org/10.1002/(SICI)1522-7243(200001/02)15:1<51::AID-BIO555>3.0.CO;2-J.
Mori K, Maki S, Niwa H, Ikedab H, Hirano T. Real light emitter in the bioluminescence of the calcium-activated photoproteins aequorin and obelin: light emission from the singlet-excited state of coelenteramide phenolate anion in a contact ion pair. Tetrahedron. 2006;62:6272–88.
Imai Y, Shibata T, Maki S, Niwa S, Ohashi M, Hirano T. Fluorescenceproperties of phenolate anion of coelenteramide analogues: the light-emitter structure in aequorin bioluminescence. J Photochem Photobiol A. 2001;146:95–107.
Hirano T, Ohmiya Y, Maki S, Niwa H, Ohashi M. Bioluminescent properties offluorinate semisynthetic aequorins. Tetrahedron. 1998;39:5541–4.
Vysotski ES, Liu ZJ, Markova SV, Blinks JR, Deng L, Frank LA, et al. Violet bioluminescence and fastkinetics from W92F obelin: structure-based proposal for the bioluminescence triggering and the identification of the emitting species. Biochemistry. 2003;42:6013–24.
Li Z-S, Zou L-Y, Min C-G, Ren A-M. The effect of micro-environment on luminescence of aequorin: the role of amino acids and explicit water molecules on spectroscopic properties of coelenteramide. J Photochem Photobiol B. 2013;127:94–9. https://doi.org/10.1016/j.jphotobiol.2013.07.022.
Min C, Li Z, Ren A, Zou L, Guo J, Goddard JD. The fluorescent properties of coelenteramide, a substrate of aequorin and obelin. J Photochem Photobiol Chem. 2013;251:182–8. https://doi.org/10.1016/j.jphotochem.2012.10.028.
Tomilin FN, Antipina LY, Vysotski ES, Ovchinnikov SG, Gitelzon II. Fluorescence of calcium-discharged obelin: the structure and molecular mechanism of emitter formation. Dokl Biochem Biophys. 2008;422:279–84. https://doi.org/10.1134/S1607672908050086.
Vysotskiĭ ES, Markova SV, Frank LA. Calcium-regulated photoproteins of marine coelenterates. Mol Biol (Mosk). 2006;40:404–17.
Frank LA. Ca2+-regulated photoproteins: effective immunoassay reporters. Sensors. 2010;10:11287–300. https://doi.org/10.3390/s101211287.
Vysotski ES, Lee J. Ca2+-regulated photoproteins: structural insight into the bioluminescence mechanism. Acc Chem Res. 2004;37:405–15. https://doi.org/10.1021/ar0400037.
Acknowledgements
The authors would like to thank Alejandro D. Arroyo, University of Pennsylvania, for critical review of the manuscript.
Funding
This work was supported by the state budget allocated to the fundamental research at the Russian Academy of Sciences, project 01201351504; the Russian Foundation for Basic Research, Grant No. 16-34-00695; and the Krasnoyarsk Regional Fund of Science and Technology Support. AVP’s research reported in this publication was supported by the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR000003.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Petrova, A.S., Lukonina, A.A., Dementyev, D.V. et al. Protein-based fluorescent bioassay for low-dose gamma radiation exposures. Anal Bioanal Chem 410, 6837–6844 (2018). https://doi.org/10.1007/s00216-018-1282-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1282-5