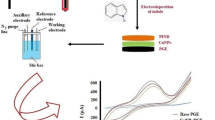
Abstract
The treatment of some inborn metabolism errors requires cholesterol substitution therapy. Cholesterol plays a vital role in the human body. Therefore, the majority of cholesterol determination techniques are targeted to blood and blood serum. Nevertheless, cholesterol determination in food is important as well. In this paper, cholesterol determination using differential pulse voltammetry (DPV) in dairy products (e.g., milk, clotted cream, yogurt, butter, etc.) is reported with a novel nonenzymatic sensor based on diphosphonic acid of 1,4-diacetylglycoluril (DPADGU) as an electrode surface modifier. Stable anodic response was obtained from cholesterol on the modified carbon-based electrode. The sensor has high stability, sensitivity (20 μA mol L−1 cm−2), and a wide linear range from 1 up to 200 μM. The LOD and LOQ values are 1.5 and 5.1 μM, respectively. The developed methods were successfully applied to the above mentioned dairy products.

ᅟ








Similar content being viewed by others
References
Korade Z, Folkes OM, Harrison FE. Behavioral and serotonergic response changes in the Dhcr7-HET mouse model of Smith–Lemli–Opitz syndrome. Pharmacol Biochem Behav. 2013;106:101–8. https://doi.org/10.1016/j.pbb.2013.03.007.
Merkens MJ, Sinden N, Brown CD, Merkens LS, Roullet J-B, Nguyen T, et al. Feeding impairments associated with plasma sterols in Smith-Lemli-Opitz syndrome. J Pediatr. 2014;165:836–41. https://doi.org/10.1016/j.jpeds.2014.06.010.
Merkens LS, Jordan JM, Penfield JA, Luetjohann D, Connor WE, Steiner RD. Plasma plant sterol levels do not reflect cholesterol absorption in children with Smith-Lemli-Opitz syndrome. J Pediatr. 2009;154:557–61. https://doi.org/10.1016/j.jpeds.2014.06.010.
Pasta S, Akhile O, Tabron D, Ting F, Shackeleton C, Watson G. Delivery of the 7-dehydrocholesterol reductase gene to the central nervous system using adeno-associated virus vector in a mouse model of Smith-Lemli-Opitz syndrome. Mol Genet Metab Rep. 2015;4:92–8. https://doi.org/10.1016/j.ymgmr.2015.07.006.
Ying L, Matabosch X, Serra M, Watson B, Shackeleton C, Watson G. Biochemical and physiological improvement in a mouse model of Smith–Lemli–Opitz syndrome (SLOS) following gene transfer with AAV vectors. Mol Genet Metab Rep. 2014;1:103–13. https://doi.org/10.1016/j.ymgmr.2014.02.002.
Becker S, Röhnike S, Empting S, Haas D, Mohnike K, Beblo S, et al. LC–MS/MS-based quantification of cholesterol and related metabolites in dried blood for the screening of inborn errors of sterol metabolism. Anal Bioanal Chem. 2015;407:5227–33. https://doi.org/10.1007/s00216-015-8731-1.
Sikora DM, Pettit-Kekel K, Penfield J, Merkens LS, Steiner RD. The near universal presence of autism spectrum disorders in children with Smith-Lemli-Opitz syndrome. Am J Med Genet. 2006;140:1511–8. https://doi.org/10.1002/ajmg.a.
Tierney E, Nwokoro NA, Porter FD, Freund LS, Ghuman JK, Kelley RI. Behavior phenotype in the RSH/Smith-Lemli-Opitz syndrome. Am J Med Genet. 2000;98:191–200. https://doi.org/10.1002/1096-8628(20010115)98:2<191::Aid-Ajmg1030>3.0.Co;2-M.
Kawamoto H, Yu O, Maekawa M, Shimada M, Mano N, Iida T. An efficient synthesis of 4α- and 4β-hydroxy- 7-dehydrocholesterol, biomarkers for patients with and animal models of the Smith–Lemli–Opitz syndrome. Chem Phys Lipids. 2013;175:73–8. https://doi.org/10.1016/j.chemphyslip.2013.07.004.
Korade Z, Xu L, Harrison FE, Ahsen R, Hart SE, Folkes OM, et al. Porter NA antioxidant supplementation ameliorates molecular deficits in Smith-Lemli-Opitz syndrome. Biol Psychiatry. 2014;75:215–22211. https://doi.org/10.1016/j.biopsych.2013.06.013.
Gonçalves Albuquerque T, Oliveira M, Sanches-Silva A, Costa HS. Cholesterol determination in foods: comparison between high performance and ultra-high performance liquid chromatography. Food Chem. 2016;193:18–25. https://doi.org/10.1016/j.foodchem.2014.09.109.
Amaral C, Gallardo E, Rodrigues R, Pinto Leite R, Quelhas D, Tomaz C, et al. Quantitative analysis of five sterols in amniotic fluid by GC–MS: application to the diagnosis of cholesterol biosynthesis defects. J Chromatogr B. 2010;878:2130–6. https://doi.org/10.1016/j.jchromb.2010.06.010.
Buszewska-Forajta M, Bujak R, Yumba-Mpanga A, Siluk D, Kaliszan R. GC/MS technique and AMDIS software application in identification of hydrophobic compounds of grasshoppers’ abdominal secretion (Chorthippusspp). J Pharm Biomed Anal. 2015;102:331–9. https://doi.org/10.1016/j.jpba.2014.09.039.
Saraiva D, Semedo R, da Conceição Castilho M, Silva JM, Ramos F. Selection of the derivatization reagent—the case of human blood cholesterol, its precursors and phytosterols GC–MS analyses. J Chromatogr B. 2011;879:3806–11. https://doi.org/10.1016/j.jchromb.2011.10.021.
Hernández D, González M, Astudillo P, Hernández L, González F. Modification of carbon electrodes by anodic oxidation of organic anions. Procedia Chem. 2014;12:3–8. https://doi.org/10.1016/j.proche.2014.12.034.
Yao C, Sun H, Fu H-F, Tan Z-C. Sensitive simultaneous determination of nitrophenol isomers at poly(p-aminobenzene sulfonic acid) film modified graphite electrode. Electrochim Acta. 2015;156:163–70. https://doi.org/10.1016/j.electacta.2015.01.043.
Oldham KB, Myland JC, Bond AM. Electrochemical science and technology: fundamentals and applications. New York: Wiley; 2012. 413 p
Wang W, Bai H, Li H, Lv Q, Zhang Q, Wang S. Carbon tape coated with gold film as stickers for bulk fabrication of disposable gold electrodes to detect Cr(VI). Sensors Actuators B Chem. 2016;236:218–25. https://doi.org/10.1016/j.snb.2016.05.155.
Sajid M, Nazal M, Mansha M, Alsharaa A, Jillani S, Basheer C. Chemically modified electrodes for electrochemical detection of dopamine in the presence of uric acid and ascorbic acid: a review. TrAC. 2016;76:15–29. https://doi.org/10.1016/j.trac.2015.09.006.
Morzycki JW, Sobkowiak A. Electrochemical oxidation of cholesterol. Beilstein J Org Chem. 2015;11:392–402. https://doi.org/10.3762/bjoc.11.45.
Shakhmaeva I, Saifullina D, Sattarova L, Abdullin T. Electrochemical sensor for blood deoxyribonucleases: design and application to the diagnosis of autoimmune thyroiditis. Anal Bioanal Chem. 2011;401(76):2591–7. https://doi.org/10.1007/s00216-011-5335-2.
Sal’keeva LK, Taishibekova EK, Bakibaev AA, Minaeva EV, Makin BK, Sugralina LM, et al. New phosphorylated Glycoluril derivatives. Russ J Gen Chem. 2017;87(3):442–6. https://doi.org/10.1134/s1070363217030124.
Noskova GN, Zakharova EA, Chernov VI, Zaichko AV, Elesova EE. Kabakaev AS fabrication and application of gold microelectrode ensemble based on carbon black–polyethylene composite electrode. Anal Methods. 2011;3(5):11–30. https://doi.org/10.1039/c1ay05074e.
Ahn J-H, Jeong I-S, Kwak B-M, Leem D, Yoon T, Yoon C, et al. Rapid determination of cholesterol in milk containing emulsified foods. Food Chem. 2012;135:2411–7. https://doi.org/10.1016/j.foodchem.2012.07.060.
Steven JT, Golovko VB, Johannessen B, Marshall AT. Electrochemical stability of carbon-supported gold nanoparticles in acidic electrolyte during cyclic voltammetry. Electrochim Acta. 2016;187:593–604. https://doi.org/10.1016/j.electacta.2015.11.096.
Gennaro A, Isse AA, Giussani E, Mussini PR, Primerano I, Rossi M. Relationship between supporting electrolyte bulkiness and dissociative electron transfer at catalytic and non-catalytic electrodes. Electrochim Acta. 2013;89:52–62. https://doi.org/10.1016/j.electacta.2012.11.013.
Pasciak EM, Hochstetler SE, Mubarak MS, Evans DS, Peters DG. Electrochemical reduction of phthalide at carbon cathodes in dimethylformamide: effects of supporting electrolyte and gas chromatographic injector-port chemistry on the product distribution. Electrochim Acta. 2013;113:557–63. https://doi.org/10.1016/j.electacta.2013.09.124.
Marichev VA. Reversibility of platinum voltammograms in aqueous electrolytes and ionic product of water. Electrochim Acta. 2008;53:7952–60. https://doi.org/10.1016/j.electacta.2008.05.076.
Wang Y, Tian L, Yao Z, Li F, Li S, Ye S. Enhanced reversibility of red phosphorus/active carbon composite as anode for lithium ion batteries. Electrochim Acta. 2015;163:71–6. https://doi.org/10.1016/j.electacta.2015.02.151.
Xu X, Feng Y, Li J, Li F, Yu H. A novel protocol for covalent immobilization of thionine on glassy carbon electrode and its application in hydrogen peroxide biosensor. Biosens Bioelectron. 2010;25:2324–8. https://doi.org/10.1016/j.bios.2010.03.027.
Magnusson B, Örnemark U (eds.) Eurachem Guide: The Fitness for Purpose of Analytical Methods – A Laboratory Guide to Method Validation and Related Topics, (2nd ed. 2014). Available from http://www.eurachem.orgMagnusson
Chen Y-Z, Kao S-Y, Jian H-C, Yu Y-M, Li J-Y, Wang W-H, et al. Determination of cholesterol and four phytosterols in foods without derivatization by gas chromatography-tandem mass spectrometry. J Food Drug Anal. 2015;23:636–44. https://doi.org/10.1016/j.jfda.2015.01.010.
Xu X-H, Li R-K, Chen J, Chen P, X-Ya L, Rao P-F. Quantification of cholesterol in foods using non-aqueous capillary electrophoresis. J Chromatogr B. 2002;768:369–73. https://doi.org/10.1016/S0378-4347(01)00539-4.
Acknowledgements
The research was partly funded from the State program «Science» (project No. 4.5752.2017) and the framework of Tomsk Polytechnic University Competitiveness Enhancement Program grant. JB thanks for financial support of Grant Agency of the Czech Republic (project P206/12/G151).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
No humans are involved in this study.
Rights and permissions
About this article
Cite this article
Derina, K., Korotkova, E., Taishibekova, Y. et al. Electrochemical nonenzymatic sensor for cholesterol determination in food. Anal Bioanal Chem 410, 5085–5092 (2018). https://doi.org/10.1007/s00216-018-1164-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1164-x