Abstract
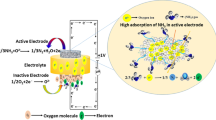
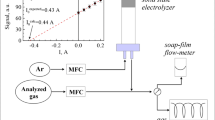
Sensor signal drift is the key issue for the reliability of continuous gas sensors. In this paper, we characterized the sensing signal drift of an amperometric ionic liquid (IL)-based oxygen sensor to identify the key chemical parameters that contribute to the signal drift. The signal drifts due to the sensing reactions of the analyte oxygen at the electrode/electrolyte interface at a fixed potential and the mass transport of the reactant and product at the electrode/electrolyte interface were systematically studied. Results show that the analyte concentration variation and the platinum electrode surface activity are major factors contributing to sensing signal drift. An amperometric method with a double potential step incorporating a conditioning step was tested and demonstrated to be useful in reducing the sensing signal drift and extending the sensor operation lifetime. Also, a mathematic method was tested to calibrate the baseline drift and sensing signal sensitivity change for continuous sensing. This study provides the understanding of the chemical processes that contribute to the IL electrochemical gas (IL-EG) sensor signal stability and demonstrates some effective strategies for signal drift calibration that can increase the reliability of the continuous amperometric sensing.

ᅟ






Similar content being viewed by others
References
Moeller DW, Moeller DW (2009) Environmental health. Harvard University Press, Cambridge, Massachusetts and London, England
Wang Z, Guo M, Baker GA, Stetter JR, Lin L, Mason AJ, et al. Methane–oxygen electrochemical coupling in an ionic liquid: a robust sensor for simultaneous quantification. Analyst. 2014;139:5140–7.
Wang Z, Lin P, Baker GA, Stetter J, Zeng X. Ionic liquids as electrolytes for the development of a robust amperometric oxygen sensor. Anal Chem. 2011;83:7066–73.
Mu X, Wang Z, Zeng X, Mason AJ. A robust flexible electrochemical gas sensor using room temperature ionic liquid. IEEE Sensors J. 2013;13:3976–81.
Xiao C, Rehman A, Zeng X. Dynamics of redox processes in ionic liquids and their interplay for discriminative electrochemical sensing. Anal Chem. 2012;84:1416–24.
Tang Y, Lin L, Kumar A, Guo M, Sevilla M, Zeng X. Hydrogen electrooxidation in ionic liquids catalyzed by the NTf2 radical. J Phys Chem C. 2017;121:5161–7.
Tang Y, Zeng X. Electrochemical oxidation of hydrogen in Bis (trifluoromethylsulfonyl) imide ionic liquids under anaerobic and aerobic conditions. J Phys Chem C. 2016;120:23542–51.
Tang Y, Wang Z, Chi X, Sevilla MD, Zeng X. In situ generated platinum catalyst for methanol oxidation via electrochemical oxidation of Bis (trifluoromethylsulfonyl) imide anion in ionic liquids at anaerobic condition. J Phys Chem C Nanomater Interfaces. 2015;120:1004–12.
Lin L, Rehman A, Chi X, Zeng X. 2, 4-Toluene diisocyanate detection in liquid and gas environments through electrochemical oxidation in an ionic liquid. Analyst. 2016;141:1519–29.
Vergara A, Vembu S, Ayhan T, Ryan MA, Homer ML, Huerta R. Chemical gas sensor drift compensation using classifier ensembles. Sensors Actuators B Chem. 2012;166:320–9.
Buzzeo MC, Hardacre C, Compton RG. Use of room temperature ionic liquids in gas sensor design. Anal Chem. 2004;76:4583–8.
Silvester DS. Recent advances in the use of ionic liquids for electrochemical sensing. Analyst. 2011;136:4871–82.
Stetter JR, Penrose WR. Understanding chemical sensors and chemical sensor arrays (electronic noses): past, present, and future. Sensors Updat. 2002;10:189.
Holmberg M, Winquist F, Lundström I, Davide F, DiNatale C, D’Amico A. Drift counteraction for an electronic nose. Sensors Actuators B Chem. 1996;36:528–35.
Dossi N, Toniolo R, Pizzariello A, Carrilho E, Piccin E, Battiston S, et al. An electrochemical gas sensor based on paper supported room temperature ionic liquids. Lab Chip. 2012;12:153–8.
Lu L, Zhao P, Mason A, Zeng X. Characterization of the IL-electrode interfacial relaxation processes under potential polarization for ionic liquid amperometric gas sensor method development. ACS Sensors. 2018. https://doi.org/10.1021/acssensors.8b00155.
Fonollosa J, Neftci E, Huerta R, Marco S. Evaluation of calibration transfer strategies between metal oxide gas sensor arrays. Procedia Eng. 2015;120:261–4. https://www.sciencedirect.com/science/article/pii/S187770581502264X
Arshak K, Moore E, Lyons GM, Harris J, Clifford S. A review of gas sensors employed in electronic nose applications. Sens Rev. 2004;24:181–98.
Li Z, Liu H, Liu Y, He P, Li J. A room temperature ionic-liquid-templated proton-conducting gelatinous electrolyte. J Phys Chem B. 2004;108:17512–8.
Liu Y, Li J, Wang M, Li Z, Liu H, He P, et al. Preparation and properties of nanostructure anatase TiO2 monoliths using 1-butyl-3-methylimidazolium tetrafluoroborate room temperature ionic liquids as template solvents. Cryst Growth Des. 2005;5:1643–9. http://pubs.rsc.org/en/content/articlelanding/2005/cc/b501224d/unauth#!divAbstract
Yu L, Garcia D, Ren R, Zeng X. Ionic liquid high temperature gas sensors. Chem Commun. 2005;0:2277–79.
Endres F, El Abedin SZ. Air and water stable ionic liquids in physical chemistry. Phys Chem Chem Phys. 2006;8:2101–16.
Xiong L, Aldous L, Henstridge MC, Compton RG. Investigation of the optimal transient times for chronoamperometric analysis of diffusion coefficients and concentrations in non-aqueous solvents and ionic liquids. Anal Methods. 2012;4:371–6.
Buzzeo MC, Klymenko OV, Wadhawan JD, Hardacre C, Seddon KR, Compton RG. Voltammetry of oxygen in the room temperature ionic liquids 1-ethyl-3-methylimidazolium bis [(trifluoromethyl) sulfonyl] imide and hexyltriethylammonium bis [(trifluoromethyl) sulfonyl] imide: one-electron reduction to form superoxide. Steady-state and tr. J Phys Chem A. 2003;107:8872–8.
Xiao C, Rehman A, Zeng X. Evaluation of the dynamic electrochemical stability of ionic liquid--metal interfaces against reactive oxygen species using an in situ electrochemical quartz crystal microbalance. RSC Adv. 2015;5:31826–36.
Campbell JL, Rustad LE, Porter JH, Taylor JR, Dereszynski EW, Shanley JB, et al. Quantity is nothing without quality: automated QA/QC for streaming environmental sensor data. Bioscience. 2013;63:574–85.
Wenzel MJ, Mensah-Brown A, Josse F, Yaz EE. Online drift compensation for chemical sensors using estimation theory. IEEE Sensors J. 2011;11:225–32.
Panchenko A, Koper MTM, Shubina TE, Mitchell SJ, Roduner E. Ab initio calculations of intermediates of oxygen reduction on low-index platinum surfaces. J Electrochem Soc. 2004;151:A2016–27.
Pandey S. Analytical applications of room-temperature ionic liquids: a review of recent efforts. Anal Chim Acta. 2006;556:38–45.
Bard AJ, Faulkner LR, et al. Fundamentals and applications. Electrochem Methods, 2nd ed. New York: Wiley; 2001.
Parsons R. The kinetics of electrode reactions and the electrode material. Surf Sci. 1964;2:418–35.
Jewell RC. Platinum in the glass industry. Platin Met Rev. 1964;8:122–7.
Adelman WJ, Palti Y. The effects of external potassium and long duration voltage conditioning on the amplitude of sodium currents in the giant axon of the squid, Loligo pealei. J Gen Physiol. 1969;54:589–606.
Aspelund A, Jordal K. Gas conditioning—the interface between CO2 capture and transport. Int J Greenh Gas Control. 2007;1:343–54.
Schwenke H, Schmitt R, Jatzkowski P, Warmann C. On-the-fly calibration of linear and rotary axes of machine tools and CMMs using a tracking interferometer. CIRP Ann Technol. 2009;58:477–80.
Hasenfratz D, Saukh O, Thiele L (2012) On-the-fly calibration of low-cost gas sensors. In: European Conference on Wireless Sensor Networks. pp. 228–244
Gutierrez-Osuna R (2003) Signal processing methods for drift compensation. In: 2nd NOSE II Workshop Linköping. pp. 18–21
Acknowledgements
X. Zeng acknowledges grant support from the National Institute of Environmental Health (R01ES022302) and the Alpha Foundation AFC518-2 for this research. The authors thank Dr. Xiaojun Liu and Dr. Jessica Koppen for helpful comments and proofreading.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest of this work.
Additional information
Published in the topical collection Ionic Liquids as Tunable Materials in (Bio)Analytical Chemistry with guest editors Jared L. Anderson and Kevin D. Clark.
Electronic supplementary material
ESM 1
(PDF 578 kb)
Rights and permissions
About this article
Cite this article
Lin, L., Zeng, X. Toward continuous amperometric gas sensing in ionic liquids: rationalization of signal drift nature and calibration methods. Anal Bioanal Chem 410, 4587–4596 (2018). https://doi.org/10.1007/s00216-018-1090-y
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1090-y