Abstract
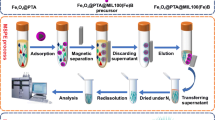
In this study, multi-walled carbon nanotubes were coated on the surface of magnetic nanoparticles modified by polydopamine. The synthesized composite was characterized and applied to magnetic-μ-dispersive solid-phase extraction of oxcarbazepine (OXC), phenytoin (PHT), and carbamazepine (CBZ) from human plasma, urine, and cerebrospinal fluid samples prior to analysis by a high-performance liquid chromatography-photodiode array detector. The extraction parameters were investigated and the optimum condition was obtained when the variables were set to the following: sorbent type, Fe3O4@polyDA–MWCNTs (length < 2 μm); sample pH, 6; amount of sorbent, 15 mg; sorption time, 1.5 min at room temperature; type and volume of the eluent, 2.5 mL methanol; and salt content, none added. Under the optimized conditions, the calibration curves are linear in the concentration range 2–2000 ng/mL, the limits of detection are in the range 0.4–3.1 ng/mL, and the relative standard deviations and relative recoveries of plasma (spiked at 200 ng/mL) and CSF (spiked at 50 ng/mL) are in the ranges 1.4–8.2% and 92.8–96.5%, respectively. The applicability of the method was successfully confirmed by extraction and determination of OXC, PHT, and CBZ in biological matrices.

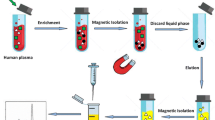
Magnetic multi-walled carbon nanotube core–shell composites were applied as magnetic-μ-dispersive solid-phase extraction adsorbents for determination of antiepileptic drugs in biological matrices.






Similar content being viewed by others
References
Nicholas JM, Ridsdale L, Richardson MP, Ashworth M, Gulliford MC. Trends in antiepileptic drug utilisation in UK primary care 1993–2008: cohort study using the general practice research database. Seizure. 2012;21(6):466–70.
Kambli L, Bhatt LK, Oza M, Prabhavalkar K. Novel therapeutic targets for epilepsy intervention. Seizure. 2017;51:27–34.
Novak PH, Ekins-Daukes S, Simpson CR, Milne RM, Helms P, McLay JS. Acute drug prescribing to children on chronic antiepilepsy therapy and the potential for adverse drug interactions in primary care. Br J Clin Pharmacol. 2005;59(6):712–7.
Esposito S, Canevini MP, Principi N. Complications associated with antibiotic administration: neurological adverse events and interference with antiepileptic drugs. Int J Antimicrob Agents. 2017;50(1):1–8.
Krasowski MD. Therapeutic drug monitoring of the newer anti-epilepsy medications. Pharmaceuticals. 2010;3(6):1909–35.
Vasconcelos I, Fernandes C. Magnetic solid phase extraction for determination of drugs in biological matrices. Trends Anal Chem. 2017;89:41–52.
Morovati A, Ahmad Panahi H, Yazdani F. Grafting of allylimidazole and n-vinylcaprolactam as a thermosensitive polymer onto magnetic nano-particles for the extraction and determination of celecoxib in biological samples. Int J Pharm. 2016;513(1–2):62–7.
Arghavani-Beydokhti S, Rajabi M, Asghari A. Combination of magnetic dispersive micro solid-phase extraction and supramolecular solvent-based microextraction followed by high-performance liquid chromatography for determination of trace amounts of cholesterol-lowering drugs in complicated matrices. Anal Bioanal Chem. 2017;409(18):1–13.
Kong XJ, Zheng C, Lan YH, Chi SS, Dong Q, Liu HL, et al. Synthesis of multirecognition magnetic molecularly imprinted polymer by atom transfer radical polymerization and its application in magnetic solid-phase extraction. Anal Bioanal Chem. 2017;410(1):247–57.
Ghorbanil M, Chamsazl M, Rounaghil GH, Aghamohammadhasani M, Seyedin O, Lahoori NA. Development of a novel ultrasonic-assisted magnetic dispersive solid-phase microextraction method coupled with high performance liquid chromatography for determination of mirtazapine and its metabolites in human urine and water samples employing experimental design. Anal Bioanal Chem. 2016;408:7719–29.
Corps Ricardo AI, Guzmán Bernardo FJ, Zougagh M, Rodríguez Martín-Doimeadios RC, Ríos A. Magnetic nanoparticles-carbon nanotubes hybrid composites for selective solid-phase extraction of polycyclic aromatic hydrocarbons and determination by ultra-high performance liquid chromatography. Anal Bioanal Chem. 2017;409(21):5125–32.
Asgharinezhad AA, Ebrahimzadeh H, Mirbabaei F, Mollazadeh N, Shekari N. Dispersive micro-solid-phase extraction of benzodiazepines from biological fluids based on polyaniline/magnetic nanoparticles composite. Anal Chim Acta. 2014;844:80–9.
Amiri M, YadollahYamini SM, Asiabi H. Magnetite nanoparticles coated with covalently immobilized ionic liquids as a sorbent for extraction of non-steroidal anti-inflammatory drugs from biological fluids. Microchim Acta. 2016;183(7):2297–305.
Dong S, Huang G, Su M, Huang T. Environmentally friendly method: development and application to carbon aerogel as sorbent for solid-phase extraction. ACS Appl Mater Interfaces. 2015;7(40):22256–63.
Zhao Q, Wei F, Luo YB, Ding J, Xiao N, Feng YQ. Rapid magnetic solid-phase extraction based on magnetic multiwalled carbon nanotubes for the determination of polycyclic aromatic hydrocarbons in edible oils. J Agric Food Chem. 2011;59(24):12794–800.
Herrero-Latorre C, Barciela-Garcia J, Garcia-Martin S, Pena-Crecente RM, Otarola-Jimenez J. Magnetic solid-phase extraction using carbon nanotubes as sorbents: a review. Anal Chim Acta. 2015;892:10–26.
Xiao D, Dramou P, Xiong N, He H, Li H, Yuan D, et al. Development of novel molecularly imprinted magnetic solid-phase extraction materials based on magnetic carbon nanotubes and their application for the determination of gatifloxacin in serum samples coupled with high performance liquid chromatography. J Chromatogr A. 2013;1274:44–53.
Daneshvar Tarigh G, Shemirani F. Simultaneous in situ derivatization and ultrasound-assisted dispersive magnetic solid phase extraction for thiamine determination by spectrofluorimetry. Talanta. 2014;123:71–7.
Demir A, Baykal A, Sözeri H, Topkaya R. Low temperature magnetic investigation of Fe3O4 nanoparticles filled into multiwalled carbon nanotubes. Synth Met. 2014;187:75–80.
Morales-Cid G, Fekete A, Simonet BM, Lehmann R, Cardenas SC, Zhang X, et al. In situ synthesis of magnetic multiwalled carbon nanotube composites for the clean-up of (fluoro)quinolones from human plasma prior to ultrahigh pressure liquid chromatography analysis. Anal Chem. 2010;82:2743–52.
Zhu J, Wei S, Gu H, Rapole SB, Wang Q, Luo Z, et al. One-pot synthesis of magnetic graphene nanocomposites decorated with core@double-shell nanoparticles for fast chromium removal. Environ Sci Technol. 2012;46(2):977–85.
Han WJ, Piao SH, Choi HJ, Seo Y. Core–shell structured mesoporous magnetic nanoparticles and their magnetorheological response. Colloids Surf A Physicochem Eng Asp. 2017;524:79–86.
Zhao M, Deng C, Zhang X. The design and synthesis of a hydrophilic core-shell-shell structured magnetic metal-organic framework as a novel immobilized metal ion affinity platform for phosphoproteome research. Chem Commun. 2014;50(47):6228–31.
Jia Y, Su H, Wong YLE, Chen X, Dominic Chan TW. Thermo-responsive polymer tethered metal-organic framework core-shell magnetic microspheres for magnetic solid-phase extraction of alkylphenols from environmental water samples. J Chromatogr A. 2016;1456:42–8.
Lee H, Dellatore SM, Miller WM, Messersmith PB. Mussel-inspired surface chemistry for multifunctional coatings. Science. 2007;318(5849):426–30.
González-Sálamo J, Socas-Rodríguez B, Hernández-Borges J, Rodríguez-Delgado MÁ. Core-shell poly(dopamine) magnetic nanoparticles for the extraction of estrogenic mycotoxins from milk and yogurt prior to LC–MS analysis. Food Chem. 2017;215:362–8.
Zhan H, Jagtiani T, Liang JF. A new targeted delivery approach by functionalizing drug nanocrystals through polydopamine coating. Eur J Pharm Biopharm. 2017;114:221–9.
Zhao C, Zhang G, Xu X, Yang F, Yang Y. Rapidly self-assembled polydopamine coating membranes with polyhexamethylene guanidine: formation, characterization and antifouling evaluation. Colloids Surf A Physicochem Eng Asp. 2017;512:41–50.
Che D, Cheng J, Ji Z, Zhang S, Li G, Sun Z, et al. Recent advances and applications of polydopamine-derived adsorbents for sample pretreatment. Trends Anal Chem. 2017;97:1–14.
Bakirci G, Yilmaz M, Babur E, Ozden D, Demirel G. Understanding the effect of polydopamine coating on catalytic reduction reactions. Catal Commun. 2017;91:48–52.
Hooshfar S, Basiri B, Bartlett MG. Development of a surrogate matrix for cerebral spinal fluid for liquid chromatography/mass spectrometry based analytical methods. Rapid Commun Mass Spectrom. 2016;30(7):854–8.
Sun Z, Wang W, Wen H, Gan C, Lei H, Liu Y. Sensitive electrochemical immunoassay for chlorpyrifos by using flake-like Fe3O4 modified carbon nanotubes as the enhanced multienzyme label. Anal Chim Acta. 2015;899:91–9.
Mei M, Huang X, Yang X, Luo Q. Effective extraction of triazines from environmental water samples using magnetism-enhanced monolith-based in-tube solid phase microextraction. Anal Chim Acta. 2016;937:69–79.
Abdollahi E, Abdouss M, Mohammadi A. Synthesis of a nano molecularly imprinted polymeric sorbent for solid phase extraction and determination of phenytoin in plasma, urine, and wastewater by HPLC. RSC Adv. 2016;6(45):39095–105.
Wong KT, Yoon Y, Snyder SA, Jang M. Phenyl-functionalized magnetic palm-based powdered activated carbon for the effective removal of selected pharmaceutical and endocrine-disruptive compounds. Chemosphere. 2016;152:71–80.
Qu L, Fan Y, Wang W, Ma K, Yin Z. Development, validation and clinical application of an online-SPE-LC-HRMS/MS for simultaneous quantification of phenobarbital, phenytoin, carbamazepine, and its active metabolite carbamazepine 10,11-epoxide. Talanta. 2016;158:77–88.
Wang L, Wang J, Zhang J, Jiang Q, Zhao L, Zhang T. Simultaneous determination of topiramate, carbamazepine, oxcarbazepine and its major metabolite in human plasma by SFC-ESI-MS/MS with polarity switching: application to therapeutic drug monitoring. Arab J Chem. 2016; https://doi.org/10.1016/j.arabjc.2016.09.016.
Fortuna A, Sousa J, Alves G, Falcao A, Soares-da-Silva P. Development and validation of an HPLC-UV method for the simultaneous quantification of carbamazepine, oxcarbazepine, eslicarbazepine acetate and their main metabolites in human plasma. Anal Bioanal Chem. 2010;397(4):1605–15.
Ferreira A, Rodrigues M, Oliveira P, Francisco J, Fortuna A, Rosado L, et al. Liquid chromatographic assay based on microextraction by packed sorbent for therapeutic drug monitoring of carbamazepine, lamotrigine, oxcarbazepine, phenobarbital, phenytoin and the active metabolites carbamazepine-10,11-epoxide and licarbazepine. J Chromatogr B. 2014;971:20–9.
Zhang J, Liu D, Meng X, Shi Y, Wang R, Xiao D, et al. Solid phase extraction based on porous magnetic graphene oxide/β-cyclodextrine composite coupled with high performance liquid chromatography for determination of antiepileptic drugs in plasma samples. J Chromatogr A. 2017;1524:49–56.
Funding
This study was financially supported by the National Natural Science Foundation of China (grant no. 21675008) and the Beijing Natural Science Foundation (grant no. 2132048).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
This is not a clinical study on humans with an ethics committee. Voluntary donors donate biological samples to the Peking Union Medical College Hospital and the samples were transferred to our laboratory. The Peking Union Medical College Hospital ensures donor-product-laboratory traceability and donor consent.
Conflict of interest
The authors declare that they have no competing interests.
Electronic supplementary material
ESM 1
(PDF 308 kb)
Rights and permissions
About this article
Cite this article
Zhang, R., Wang, S., Yang, Y. et al. Modification of polydopamine-coated Fe3O4 nanoparticles with multi-walled carbon nanotubes for magnetic-μ-dispersive solid-phase extraction of antiepileptic drugs in biological matrices. Anal Bioanal Chem 410, 3779–3788 (2018). https://doi.org/10.1007/s00216-018-1047-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1047-1