Abstract
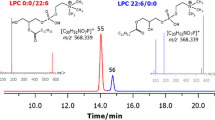
The task of lipid analysis and profiling is taking centre stage in many research fields and as a consequence, there has been an intense effort to develop suitable methodologies to discover, identify, and quantify lipids in the systems investigated. Given the high complexity and diversity of the lipidome, researchers have been challenged to afford thorough knowledge of all the lipid species in a given sample, by gathering the data obtained by complementary analytical techniques. In this research, an “omic” approach was developed to quickly fingerprint lipids in the Mediterranean mussel (Mytilus galloprovincialis), by exploiting multidimensional and hyphenated techniques. In detail, two-dimensional comprehensive hydrophilic interaction liquid chromatography coupled to reversed-phase liquid chromatography afforded both class-type separation and lipid assignment within the total lipid species in the sample, by the coupling of a 2.1-mm I.D. partially porous stationary phase in the first dimension, to a short (50 mm) monodisperse octadecylsilica secondary column; individual molecular species were afterwards identified by means of their ion trap-time of flight mass spectra obtained by electrospray ionization. More than 200 neutral and polar lipids were identified, and among the latter, phosphatydylcholine and phosphatydylethanolamine were the most represented classes, together with their mono-acylated forms, plasmanyl and plasmenyl derivatives. Subsequently, separation of the saturated and unsaturated isomers of the fatty acids (including the saturated C16:0 and the polyunsaturated C22:6) in the offline collected phospholipid fractions was accomplished by gas chromatography analysis of the corresponding methyl esters, on a 200 m × 0.25 mm, 0.2 μm d f ionic liquid column.


Similar content being viewed by others
References
Teslovich TM, Musunuru K, …, Kathiresan S. Biological, clinical and population relevance of 95 loci for blood lipids. Nature. 2010;466:707–13.
Akoh CC. Food lipids: chemistry, nutrition, and biotechnology. 4th ed. Boca Raton: CRC Press; 2017.
Hyötyläinen T, Orešič M. Optimizing the lipidomics workflow for clinical studies—practical considerations. Anal Bioanal Chem. 2015;407:4973–93.
Donato P, Cacciola F, Beccaria M, Dugo P, Mondello L. Lipidomics. In: Picò Y, editor. Advanced mass spectrometry for food safety and quality. Amsterdam: Elsevier; 2015. p. 395–439.
Donato P, Inferrera I, Sciarrone D, Mondello L. Supercritical fluid chromatography for lipid analysis in foodstuffs. J Sep Sci. 2017;40:361–82.
Beccaria M, Sullini G, Cacciola F, Donato P, Dugo P, Mondello L. High performance characterization of triacylglycerols in milk and milk-related samples by liquid chromatography and mass spectrometry. J Chromatogr A. 2014;1360:172–87.
Lísa M, Holčapek M. Triacylglycerols profiling in plant oils important in food industry, dietetics and cosmetics using high performance liquid chromatography-atmospheric pressure chemical ionization mass spectrometry. J Chromatogr A. 2008;1198:115–30.
Hutchins PM, Barkley RM, Murphy RC. Separation of cellular non polar neutral lipids by normal-phase chromatography and analysis by electrospray ionization mass spectrometry. J Lipid Res. 2008;49:804–13.
Donato P, Cacciola F, Cichello F, Russo M, Dugo P, Mondello L. Determination of phospholipids in milk samples by means of hydrophilic interaction liquid chromatography coupled to evaporative light scattering and mass spectrometry detection. J Chromatogr A. 2011;1218:6476–82.
Tranchida PQ, Donato P, Dugo P, Dugo G, Mondello L. Comprehensive chromatographic methods for the analysis of lipids. TrAC Trends Anal Chem. 2007;26:191–205.
Jandera P. Column selectivity for two-dimensional liquid chromatography. J Sep Sci. 2006;29:1763–83.
Jandera P. Comprehensive two-dimensional liquid chromatography—practical impacts of theoretical considerations. A review. Cent Eur J Chem. 2012;10:844–75.
Dugo P, Fawzy N, Cichello F, Cacciola F, Donato P, Mondello L. Stop-flow comprehensive two-dimensional liquid chromatography combined with mass spectrometric detection for phospholipid analysis. J Chromatogr A. 2013;1278:46–53.
Lísa M, Cífková E, Holčapek M. Lipidomic profiling of biological tissues using off-line two-dimensional high-performance liquid chromatography-mass spectrometry. J Chromatogr A. 2011;1218:5146–56.
Li M, Tong X, Lv P, Feng B, Yang L, Wu Z, et al. A not-stop-flow online normal-/reversed-phase two-dimensional liquid chromatography–quadrupole time-of-flight mass spectrometry method for comprehensive lipid profiling of human plasma from atherosclerosis patients. J Chromatogr A. 2014;1372:110–9.
Donato P, Cacciola F, Tranchida PQ, Dugo P, Mondello L. Mass spectrometry detection in comprehensive liquid chromatography: basic concepts, instrumental aspects, applications and trends. Mass Spectrom Rev. 2012;31:523–59.
Holčapek M, Ovčačíková M, Lísa M, Cífková E, Hájek T. Continuous comprehensive two-dimensional liquid chromatography–electrospray ionization mass spectrometry of complex lipidomic samples. Anal Bioanal Chem. 2015;407:5033–43.
Baglai A, Gargano AFG, Jordens J, Mengerink Y, Honing M, van der Wal S, et al. Comprehensive lipidomic analysis of human plasma using multidimensional liquid- and gas-phase separations: two-dimensional liquid chromatography-mass spectrometry vs. liquid chromatography-trapped-ion-mobility-mass spectrometry. J Chromatogr A. 2017;1530:90–103.
Cajka T, Fiehn P. Comprehensive analysis of lipids in biological systems by liquid chromatography-mass spectrometry. TrAC Trends Anal Chem. 2014;61:192–206.
Zoccali M, Schug KA, Walsh P, Smuts J, Mondello L. Flow-modulated comprehensive two-dimensional gas chromatography combined with a vacuum ultraviolet detector for the analysis of complex mixtures. J Chromatogr A. 2017;1497:135–43.
Delmonte P, Fardin-Kia AR, Rader JI. Separation of fatty acid methyl esters by GC-online hydrogenation×GC. Anal Chem. 2013;85:1517–24.
Anderson JL, Armstrong DW. High-stability ionic liquids. A new class of stationary phases for gas chromatography. Anal Chem. 2003;75:4851–8.
Amaral MSS, Marriott PJ, Bizzo HR, et al. Ionic liquid capillary columns for analysis of multi-component volatiles by gas chromatography-mass spectrometry: performance, selectivity, activity and retention indices. Anal Bioanal Chem. 2017; https://doi.org/10.1007/s00216-017-0718-7.
Delmonte P, Fardin-Kia AR, Kramer JKG, Mossoba MM, Sidisky L, Rader J. Separation characteristics of fatty acid methyl esters using SLB-IL111, a new ionic liquid coated capillary gas chromatographic column. J Chromatogr A. 2011;1218:545–54.
Zapadlo M, Krupcik J, Májek P, Armstrong DW, Sandra P. Use of a polar ionic liquid as second column for the comprehensive two-dimensional GC separation of PCBs. J Chromatogr A. 2010;1217:5859–67.
Seeley JV, Seeley SK, Libby EK, Breitbach ZS, Armstrong DW. Comprehensive two-dimensional gas chromatography using a high-temperature phosphonium ionic liquid column. Anal Bioanal Chem. 2008;390:323–32.
Fanali C, Micalizzi G, Dugo P, Mondello L. Ionic liquids as stationary phases for fatty acid analysis by gas chromatography. Analyst. 2017; https://doi.org/10.1039/c7an01338h.
Albergamo A, Rigano F, Purcaro G, Mauceri A, Fasulo S, Mondello L. Free fatty acid profiling of marine sentinels by nanoLC-EI-MS for the assessment of environmental pollution effects. Sci Total Environ. 2016;571:955–62.
Martínez-Pita I, Sánchez-Lazo C, Ruíz-Jarabo R, Herrera M, Mancera JM. Biochemical composition, lipid classes, fatty acids and sexual hormones in the mussel Mytilus galloprovincialis from cultivated populations in south Spain. Aquaculture. 2012;358-359:274–83.
Rigano F, Albergamo A, Sciarrone D, Beccaria M, Purcaro G, Mondello L. Nano liquid chromatography directly coupled to electron ionization mass spectrometry for free fatty acid elucidation in mussel. Anal Chem. 2016;88:4021–8.
Bligh EG, Dyer WG. A rapid method of total lipid extraction and purification. Can J Biochem Phys. 1959;37:911–7.
OECD. Test no. 305: bioaccumulation in fish: aqueous and dietary exposure. Paris: OECD Publishing; 2012. https://doi.org/10.1787/9789264185296-en.
Zhu C, Dane A, Spijksma G, Wang M, van der Greef J, Luo G, et al. An efficient hydrophilic interaction liquid chromatography separation of 7 phospholipid classes based on a diol column. J Chromatogr A. 2012;1220:26–34.
Jandera P. Programmed elution in comprehensive two-dimensional liquid chromatography. J Chromatogr A. 2012;1255:112–29.
Facchini L, Losito I, Cataldi TR, Palmisano F. Seasonal variations in the profile of main phospholipids in Mytilus galloprovincialis mussels: a study by hydrophilic interaction liquid chromatography-electrospray ionization Fourier transform mass spectrometry. J Mass Spectrom. 2017; https://doi.org/10.1002/jms.4029.
Facchini L, Losito I, Cataldi TR, Palmisano F. Ceramide lipids in alive and thermally stressed mussels: an investigation by hydrophilic interaction liquid chromatography-electrospray ionization Fourier transform mass spectrometry. J Mass Spectrom. 2016;51:768–81.
Jandera P, Hájek T, Cesla P. Comparison of various second-dimension gradient types in comprehensive two-dimensional liquid chromatography. J Sep Sci. 2010;33:1382–97.
Pirok BWJ, Gargano AFG, Schoenmakers PJ. Optimizing separations in online comprehensive two-dimensional liquid chromatography. J Sep Sci. 2017:1–30. https://doi.org/10.1002/jssc.201700863.
Donato P, Rigano F, Cacciola F, Schure M, Farnetti S, Russo M, et al. Comprehensive two-dimensional liquid chromatography–tandem mass spectrometry for the simultaneous determination of wine polyphenols and target contaminants. J Chromatogr A. 2016;1458:54–62.
Murphy KJ, Mooney BD, Mann NJ, Nichols PD, Sinclair AJ. Lipid, FA, and sterol composition of New Zealand green lipped mussel (Pernacanaliculus) and Tasmanian blue mussel (Mytilusedulis). Lipids. 2002;37:587–95.
Gorinstein S, Moncheva S, Katrich E, Toledo F, Arancibia P, Goshev I, et al. Antioxidants in the black mussel (Mytilus galloprovincialis) as an indicator of black sea coastal pollution. Mar Pollut Bull. 2003;46:1317–25.
Delmonte P, Fardin-Kia AR, Kramer JKG, Mossoba MM, Sidisky L, Tyburczy C, et al. Evaluation of highly polar ionic liquid gas chromatographic column for the determination of the fatty acids in milk fat. J Chromatogr A. 2012;1233:137–46.
Christie WW. Preparation of ester derivatives of fatty acids for chromatographic analysis. In: Christie WW, editor. Advances in lipid methodology–two. Dundee: Oily Press; 1993. p. 69–111.
Marinetti GV. Hydrolysis of lecithin with sodium methoxide. Biochemistry. 1962;1:350–3.
Marinetti GV. Low temperature partial alcoholysis of triglycerides. J Lipid Res. 1966;7:786–8.
Acknowledgments
The authors gratefully acknowledge Shimadzu Corporation and Millipore Sigma/Supelco Corporation for the continuous support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Statement of human and animal rights
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures were in accordance with guidelines for the protection of animal welfare, in compliance with the Italian National Bioethics Committee (INBC) (European Community Council Directive of November 24, 1986-86/609/EEC).
Additional information
Published in the topical collection Euroanalysis XIX with guest editors Charlotta Turner and Jonas Bergquist.
Electronic supplementary material
ESM 1
(PDF 850 kb)
Rights and permissions
About this article
Cite this article
Donato, P., Micalizzi, G., Oteri, M. et al. Comprehensive lipid profiling in the Mediterranean mussel (Mytilus galloprovincialis) using hyphenated and multidimensional chromatography techniques coupled to mass spectrometry detection. Anal Bioanal Chem 410, 3297–3313 (2018). https://doi.org/10.1007/s00216-018-1045-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-1045-3