Abstract
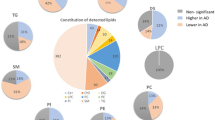
Early diagnosis of neural changes causing cognitive impairment is critical for development of preventive therapies for dementia. Biomarkers currently characterized cannot be extensively applied due to the invasive sampling of cerebrospinal fluid. The other imaging approaches are either expensive or require a high technique. Phospholipids (PLs), which are basic constituents of neurons, might be a key variable in the pathogenesis of cognitive impairment. Changes in plasma PL provide the possibility for development of novel biomarkers with minimal invasion and high patient acceptance. In this work, a HILIC-ESI-IT-TOF-MS system was introduced for untargeted profiling of plasma PLs to investigate the relationship between changes of plasma PL profiles and cognitive impairment. A total of 272 types of PL molecular structures were characterized in human plasma and quantified through the internal standard method. Univariate analysis shows 29 PLs were significantly different between the control (n = 41) and the cognitive impairment (CI) group (n = 41). Multivariate analysis (PCA and OPLS-DA) was conducted based on these 29 potential PL biomarkers. Both univariate and multivariate analyses show abnormality of PL metabolism in the CI group, and the downregulation of ethanolamine plasmalogen (pPE) supply, especially those with PUFAs, in the circulation system should be strongly associated with neurodegeneration. A discriminative model was established with satisfied fit (R2) and prediction (Q2) abilities, and the classification test showed better recognition of the CI group than the control group indicating that this model of PL biomarkers could be used as indicators for screening of CI.

Characterization of potential plasma biomarkers related to cognitive impairment by untargeted profiling of phospholipids





Similar content being viewed by others
References
International WHOaAsD. Dementia: a public health priority. 2012.
Laurin D, Verreault R, Lindsay J, MacPherson K, Rockwood K. Physical activity and risk of cognitive impairment and dementia in elderly persons. Arch Neurol. 2001;58(3):498–504.
Morris JC, Price JL. Pathologic correlates of nondemented aging, mild cognitive impairment, and early-stage Alzheimer’s disease. J Mol Neurosci. 2001;17(2):101–18.
Thal LJ. Prevention of Alzheimer disease. Alzheimer Dis Assoc Disord. 2006;20(3 Suppl 2):S97–9.
Blennow K, de Leon MJ, Zetterberg H. Alzheimer’s disease. Lancet. 2006;368(9533):387–403.
Rowe CC, Villemagne VL. Brain amyloid imaging. J Nucl Med. 2011;52(11):1733–40.
Schmidt D, Jiang Q-X, MacKinnon R. Phospholipids and the origin of cationic gating charges in voltage sensors. Nature. 2006;444(7120):775–9.
Lemmon MA. Membrane recognition by phospholipid-binding domains. Nat Rev Mol Cell Biol. 2008;9(2):99–111.
Dennis EA, Cao J, Hsu Y-H, Magrioti V, Kokotos G. Phospholipase A(2) enzymes: physical structure, biological function, disease implication, chemical inhibition, and therapeutic intervention. Chem Rev. 2011;111(10):6130–85.
Igarashi M, Ma K, Gao F, Kim H-W, Rapoport SI, Rao JS. Disturbed choline plasmalogen and phospholipid fatty acid concentrations in Alzheimer disease prefrontal cortex. J Alzheimers Dis. 2011;24(3):507–17.
Quazi F, Molday RS. Differential phospholipid substrates and directional transport by ATP-binding cassette proteins ABCA1, ABCA7, and ABCA4 and disease-causing mutants. J Biol Chem. 2013;288(48):34414–26.
Wang Y, Cella M, Mallinson K, Ulrich JD, Young KL, Robinette ML, et al. TREM2 lipid sensing sustains the microglial response in an Alzheimer’s disease model. Cell. 2015;160(6):1061–71.
Zhou Q, Lee G-S, Brady J, Datta S, Katan M, Sheikh A, et al. A hypermorphic missense mutation in PLCG2, encoding phospholipase Cγ2, causes a dominantly inherited autoinflammatory disease with immunodeficiency. Am J Hum Genet. 2012;91(4):713–20.
Min HK, Kong G, Moon MH. Quantitative analysis of urinary phospholipids found in patients with breast cancer by nanoflow liquid chromatography–tandem mass spectrometry: II. Negative ion mode analysis of four phospholipid classes. Anal Bioanal Chem. 2010;396(3):1273–80.
Cífková E, Holčapek M, Lísa M, Vrána D, Gatěk J, Melichar B. Determination of lipidomic differences between human breast cancer and surrounding normal tissues using HILIC-HPLC/ESI-MS and multivariate data analysis. Anal Bioanal Chem. 2015;407(3):991–1002.
Sommer U, Herscovitz H, Welty FK, Costello CE. LC-MS-based method for the qualitative and quantitative analysis of complex lipid mixtures. J Lipid Res. 2006;47(4):804–14.
Calderon-Santiago M, Priego-Capote F, Galache-Osuna JG, Luque de Castro MD. Analysis of serum phospholipid profiles by liquid chromatography-tandem mass spectrometry in high resolution mode for evaluation of atherosclerotic patients. J Chromatogr A. 2014;1371:154–62.
van Dongen WD, Niessen WMA. LC–MS systems for quantitative bioanalysis. Bioanalysis. 2012;4(19):2391–9.
Cífková E, Holčapek M, Lísa M, Ovčačíková M, Lyčka A, Lynen F, et al. Nontargeted quantitation of lipid classes using hydrophilic interaction liquid chromatography–electrospray ionization mass spectrometry with single internal standard and response factor approach. Anal Chem. 2012;84(22):10064–70.
Yan X, Li H, Xu J, Zhou C. Analysis of phospholipids in microalga Nitzschia closterium by UPLC-Q-TOF-MS. Chin J Oceanol Limnol. 2010;28(1):106–12.
Sato Y, Nakamura T, Aoshima K, Oda Y. Quantitative and wide-ranging profiling of phospholipids in human plasma by two-dimensional liquid chromatography/mass spectrometry. Anal Chem. 2010;82(23):9858–64.
Zhao Y-Y, Xiong Y, Curtis JM. Measurement of phospholipids by hydrophilic interaction liquid chromatography coupled to tandem mass spectrometry: the determination of choline containing compounds in foods. J Chromatogr A. 2011;1218(32):5470–9.
Walczak J, Bocian S, Buszewski B. Two-dimensional high performance liquid chromatography-mass spectrometry for phosphatidylcholine analysis in egg yolk. Food Anal Method. 2015;8(3):661–7.
Bang DY, Ej A, Moon MH. Shotgun analysis of phospholipids from mouse liver and brain by nanoflow liquid chromatography/tandem mass spectrometry. J Chromatogr B. 2007;852(1–2):268–77.
Lee JY, Min HK, Moon MH. Simultaneous profiling of lysophospholipids and phospholipids from human plasma by nanoflow liquid chromatography-tandem mass spectrometry. Anal Bioanal Chem. 2011;400(9):2953–61.
Kostiainen R, Kauppila TJ. Effect of eluent on the ionization process in liquid chromatography–mass spectrometry. J Chromatogr A. 2009;1216(4):685–99.
Zhu C, Dane A, Spijksma G, Wang M, van der Greef J, Luo G, et al. An efficient hydrophilic interaction liquid chromatography separation of 7 phospholipid classes based on a diol column. J Chromatogr A. 2012;1220:26–34.
Nasreddine ZS, Phillips NA, Bedirian V, Charbonneau S, Whitehead V, Collin I, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–9.
Hsu F-F, Turk J. Electrospray ionization with low-energy collisionally activated dissociation tandem mass spectrometry of glycerophospholipids: mechanisms of fragmentation and structural characterization. J Chromatogr B. 2009;877(26):2673–95.
Frega NG, Pacetti D, Boselli E. Characterization of phospholipid molecular species by means of HPLC-tandem mass spectrometry. In: Prasain J, editor. Tandem mass spectrometry—applications and principles. Rijeka: InTech; 2012.
Myers DS, Ivanova PT, Milne SB, Brown HA. Quantitative analysis of glycerophospholipids by LC–MS: acquisition, data handling, and interpretation. Biochim Biophys Acta. 2011;1811(11):748–57.
Goodenowe DB, Cook LL, Liu J, Lu Y, Jayasinghe DA, Ahiahonu PWK, et al. Peripheral ethanolamine plasmalogen deficiency: a logical causative factor in Alzheimer’s disease and dementia. J Lipid Res. 2007;48(11):2485–98.
Hemström P, Irgum K. Hydrophilic interaction chromatography. J Sep Sci. 2006;29(12):1784–821.
Schwalbe-Herrmann M, Willmann J, Leibfritz D. Separation of phospholipid classes by hydrophilic interaction chromatography detected by electrospray ionization mass spectrometry. J Chromatogr A. 2010;1217(32):5179–83.
Han X. Lipid alterations in the earliest clinically recognizable stage of Alzheimers disease: implication of the role of lipids in the pathogenesis of Alzheimers disease. Curr Alzheimer Res. 2005;2(1):65–77.
Katsel P, Li C, Haroutunian V. Gene expression alterations in the sphingolipid metabolism pathways during progression of dementia and Alzheimer’s disease: a shift toward ceramide accumulation at the earliest recognizable stages of Alzheimer’s disease? Neurochem Res. 2007;32(4):845–56.
Acknowledgements
We specially acknowledge the help of Shimadzu (China) with the MS technical support.
Funding
This work was supported by the Natural Science Foundation of China (81372991) and the youth fund of National Institute for Nutrition and Health (NINH2016002).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Involvement of human participants and informed consent
Human participants were volunteers from two communities. Human blood samples were collected from volunteers in this study. This work was performed with the informed consent signed by all the volunteers. The study was approved by The Ethics Committee of National Institute for Nutrition and Health and performed in accordance of the ethical standards.
Electronic supplementary material
ESM 1
(PDF 878 kb)
Rights and permissions
About this article
Cite this article
Song, S., Cheong, LZ., Man, QQ. et al. Characterization of potential plasma biomarkers related to cognitive impairment by untargeted profiling of phospholipids using the HILIC-ESI-IT-TOF-MS system. Anal Bioanal Chem 410, 2937–2948 (2018). https://doi.org/10.1007/s00216-018-0975-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0975-0