Abstract
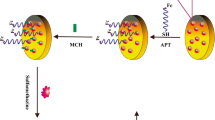
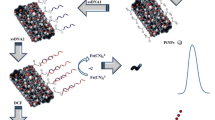
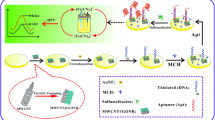
In the current study, a sensitive electrochemical sensing strategy based on aptamer (APT) for detection of sulfadimidine (SM2) was developed. A bare gold electrode (AuE) was first modified with 2-aminoethanethiol (2-AET) through self-assembly, used as linker for the subsequent immobilization of multi-walled carbon nanotubes and gold nanoparticle composites (MWCNTs/AuNPs). Then, the thiolated APT was assembled onto the electrode via sulfur-gold affinity. When SM2 existed, the APT combined with SM2 and formed a complex structure. The specific binding of SM2 and APT increased the impedance, leading to hard electron transfer between the electrode surface and the redox probe [Fe(CN)6]3−/4− and producing a significant reduction of the signal. The SM2 concentration could be reflected by the current difference of the peak currents before and after target binding. Under optimized conditions, the linear dynamic range is from 0.1 to 50 ng mL−1, with a detection limit of 0.055 ng mL−1. The sensor exhibited desirable selectivity against other sulfonamides and performs successfully when analyzing SM2 in pork samples.

A new electrochemical biosensor for ultrasensitive detection of sulfadimidine (SM2) by using a gold electrode modified with MWCNTs/AuNPs for signal amplification and aptamer (APT) for selectivity improvement.










Similar content being viewed by others
References
Qiu JR, Zhao T, Liu QY, He JH, He DC, Wu GY, et al. Residual veterinary antibiotics in pig excreta after oral administration of sulfonamides. Environ Geochem Health. 2016;38:549–56.
Bailey C, Spielmeyer A, Hamscher G, Schuttrumpf H, Frings RM. The veterinary antibiotic journey: comparing the behaviour of sulfadiazine, sulfamethazine, sulfamethoxazole and tetracycline in cow excrement and two soils. J Soils Sediments. 2016;16:1690–704.
Biosic M, Mitrevski M, Babic S. Environmental behavior of sulfadiazine, sulfamethazine, and their metabolites. Environ Sci Pollut Res. 2017;24:9802–12.
European Commission. Regulation (EU) No 37/2010 on pharmacologically active substances and their classification regarding maximum residue limits in foodstuffs of animal origin. Off J Eur Union L. 2009;15:1–72.
Nebot C, Regal P, Miranda JM, Fente C, Cepeda A. Rapid method for quantification of nine sulfonamides in bovine milk using HPLC/MS/MS and without using SPE. Food Chem. 2013;141:2294–9.
Wu HZ, Qian MR, Wang JM, Zhang H, Ma JW, Li ZG, et al. Simultaneous determination of sulfonamides and metabolites in manure samples by one-step ultrasound/microwave-assisted solid–liquid–solid dispersive extraction and liquid chromatography–mass spectrometry. Anal Bioanal Chem. 2015;407:3545–54.
Kung TA, Tsai CW, Ku BC, Wang WH. A generic and rapid strategy for determining trace multiresidues of sulfonamides in aquatic products by using an improved QuEChERS method and liquid chromatography–electrospray quadrupole tandem mass spectrometry. Food Chem. 2015;175:189–96.
Jansomboon W, Boontanon SK, Boontanon N, Polprasert C, Da CT. Monitoring and determination of sulfonamide antibiotics (sulfamethoxydiazine, sulfamethazine, sulfamethoxazole and sulfadiazine) in imported Pangasius catfish products in Thailand using liquid chromatography coupled with tandem mass spectrometry. Food Chem. 2016;212:635–40.
Xia L, Liu LJ, Lv XX, Qu F, Li GL, You JM. Towards the determination of sulfonamides in meat samples: a magnetic and mesoporous metal-organic framework as an efficient sorbent for magnetic solid phase extraction combined with high-performance liquid chromatography. J Chromatogr A. 2017;1500:24–31.
Deng KJ, Lan XH, Sun G, Ji LY, Zheng XL. Determination of sulfonamide residues in chicken liver using high-performance liquid chromatography. Food Anal Methods. 2016;9:3337–44.
Summa S, Magro SL, Armentano A, Muscarella M. Development and validation of an HPLC/DAD method for the determination of 13 sulphonamides in eggs. Food Chem. 2015;187:477–84.
Chen C, Zhang XF, Long Z, Zhang JY, Zheng CB. Molecularly imprinted dispersive solid-phase microextraction for determination of sulfamethazine by capillary electrophoresis. Microchim Acta. 2012;178:293–9.
Ruiz CG, Marina ML. Recent advances in the analysis of antibiotics by capillary electrophoresis. Electrophoresis. 2006;27:266–82.
Ma MF, Wen K, Beier RC, Eremin SA, Li CL, Zhang SX, et al. Chemiluminescence resonance energy transfer competitive immunoassay employing hapten-functionalized quantum dots for the detection of sulfamethazine. ASC Appl Mater Interfaces. 2016;8:17745–50.
Liang X, Ni HJ, Beier RC, Dong YN, Li JY, Luo XS, et al. Highly broad-specific and sensitive enzyme-linked immunosorbent assay for screening sulfonamides: assay optimization and application to milk samples. Food Anal Methods. 2014;7:1992–2002.
Pastor-Navarro N, Gallego-Iglesias E, Maquieira A, Puchades R. Immunochemical method for sulfasalazine determination in human plasma. Anal Chim Acta. 2007;583:377–83.
Hao XJ, Zhou XH, Zhang Y, Long F, Song L, Shi HC. Portable and reusable optofluidics-based biosensing platform for ultrasensitive detection of sulfadimidine in dairy products. Sensors. 2015;15:8302–13.
Tuerk C, Gold L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science. 1990;249:505–10.
Ellington AD, Szostak JW. In vitro selection of RNA molecules that bind specific ligands. Nature. 1990;346:818–22.
Song SP, Wang LH, Li J, Zhao JL, Fan CH. Aptamer-based biosensors. TrAC Trend Anal Chem. 2008;27:108–17.
Luo ZW, Wang YM, Lu XY, Chen JM, Wei FJ, Huang ZJ, et al. Fluorescent aptasensor for antibiotic detection using magnetic bead composites coated with gold nanoparticles and a nicking enzyme. Anal Chim Acta. 2017;984:177–84.
Wang AC, Zhao HM, Chen XC, Tan B, Zhang YB, Quan X. A colorimetric aptasensor for sulfadimethoxine detection based on peroxidase-like activity of graphene/nickel@palladium hybrids. Anal Biochem. 2017;525:92–9.
Chen AL, Jiang XL, Zhang WW, Chen G, Zhao Y, Tunio TM, et al. High sensitive rapid visual detection of sulfadimethoxine by label-free aptasensor. Biosens Bioelectron. 2013;42:419–25.
Kim YJ, Kim YS, Niazi JH, Gu MB. Electrochemical aptasensor for tetracycline detection. Bioprocess Biosyst Eng. 2010;33:31–7.
Liu ZY, Yuan R, Chai YQ, Zhuo Y, Hong CL, Yang X. Highly sensitive, reusable electrochemical aptasensor for adenosine. Electrochim Acta. 2009;54:6207–11.
Yang K, Xing BS. Desorption of polycyclic aromatic hydrocarbons from carbon nanomaterials in water. Environ Pollut. 2007;145:529–37.
Wei JM, Sun WL, Pan WY, Yu XQ, Sun G, Jiang H. Comparing the effects of different oxygen-containing functional groups on sulfonamides adsorption by carbon nanotubes: experiments and theoretical calculation. Chem Eng J. 2017;312:167–79.
Hrapovic S, Liu Y, Male KB, Luong JHT. Electrochemical biosensing platforms using platinum nanoparticles and carbon nanotubes. Anal Chem. 2004;76:1083–8.
Zheng TY, Bott S, Huo Q. Techniques for accurate sizing of gold nanoparticles using dynamic light scattering with particular application to chemical and biological sensing based on aggregate formation. ACS Appl Mater Interfaces. 2016;8:21585–94.
Magar HS, Ghica ME, Abbas MN, Brett CMA. A novel sensitive amperometric choline biosensor based on multi walled carbon nanotubes and gold nanoparticles. Talanta. 2017;167:462–9.
Deiminiat B, Rounaghi GH, Arbab-Zavar MH, Razavipanah I. A novel electrochemical aptasensor based on f-MWCNTs/AuNPs nanocomposite for label-free detection of bisphenol A. Sensors Actuators B Chem. 2017;242:158–66.
Taghdisi SM, Danesh NM, Emrani AS, Ramezani M, Abnous K. A novel electrochemical aptasensor based on single-walled carbon nanotubes, gold electrode and complimentary strand of aptamer for ultrasensitive detection of cocaine. Biosens Bioelectron. 2015;73:245–50.
Fei AR, Liu Q, Huan J, Qian J, Dong XY, Qiu B, et al. Label-free impedimetric aptasensor for detection of femtomole level acetamiprid using gold nanoparticles decorated multi walled carbon nanotube-reduced graphene oxide nanoribbon composites. Biosens Bioelectron. 2015;70:122–9.
Kangkamano T, Numnuam A, Limbut W, Kanatharana P, Thavarungkul P. Chitosan cryogel with embedded gold nanoparticles decorated multi walled carbon nanotubes modified electrode for highly sensitive flow based non-enzymatic glucose sensor. Sensors Actuators B Chem. 2017;246:854–63.
He BS, Zhang JX. Rapid detection of ascorbic acid based on a dual-electrode sensor system using a powder microelectrode embedded with carboxyl multi-walled carbon nanotubes. Sensors. 2017;17:1549–61.
Gaudin V, Pavy ML. Determination of sulfamethazine in milk by biosensor immunoassay. J AOAC Int. 1999;82:1316–20.
Mccaughey WJ, Elliott CT, Crooks SRH. Determination of sulphadimidine in animal feedstuffs by an enzyme-linked immunoassay. Food Addit Contam B. 1990;7:259–64.
Liu LH, Zhou XH, Xu WQ, Song BD, Shi HC. Highly sensitive detection of sulfadimidine in water and dairy products by means of an evanescent wave optical biosensor. RSC Adv. 2014;4:60227–33.
Funding
This work was supported by the National Natural Science Foundation of China (Grant No. 61301037), Henan Science and Technology Cooperation Project (Grant No. 172106000014), Cultivation Plan for Young Core Teachers in Universities of Henan Province (No. 2017GGJS072), Foundation of Henan Educational Committee (Grant No. 13A550194), Fundamental Research Funds for the Henan Provincial Colleges and Universities (Grant No. 2014YWQQ05), and Youth Backbone Teacher Training Program of Henan University of Technology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict of interest.
Rights and permissions
About this article
Cite this article
He, B., Du, G. Novel electrochemical aptasensor for ultrasensitive detection of sulfadimidine based on covalently linked multi-walled carbon nanotubes and in situ synthesized gold nanoparticle composites. Anal Bioanal Chem 410, 2901–2910 (2018). https://doi.org/10.1007/s00216-018-0970-5
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0970-5