Abstract
Quantification of cardiac troponin I (cTnI), a protein biomarker used for diagnosing myocardial infarction, has been achieved in native patient plasma based on an immunoaffinity enrichment strategy and isotope dilution (ID) liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. The key steps in the workflow involved isolating cTnI from plasma using anti-cTnI antibody coupled to magnetic nanoparticles, followed by an enzymatic digestion with trypsin. Three tryptic peptides from cTnI were monitored and used for quantification by ID-LC-MS/MS via multiple reaction monitoring (MRM). Measurements were performed using a matrix-matched calibration system. NIST SRM 2921 Human Cardiac Troponin Complex acted as the calibrant and a full-length isotopically labeled protein analog of cTnI was used as an internal standard. The method was successfully demonstrated on five patient plasma samples, with cTnI concentrations measuring between 4.86 μg/L and 11.3 μg/L (signifying moderate myocardial infarctions). LC-MS/MS measurement precision was validated by three unique peptides from cTnI and two MRM transitions per peptide. Relative standard deviation (CV) from the five plasma samples was determined to be ≤14.3%. This study has demonstrated that quantification of cTnI in native plasma from myocardial infarction patients can be achieved based on an ID-LC-MS/MS method. The development of an ID-LC-MS/MS method for cTnI in plasma is a first step for future certification of matrix-based reference materials, which may be used to help harmonize discordant cTnI clinical assays.

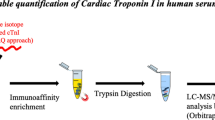
A schematic of the workflow for measuring cardiac troponin I (cTnI), a low-abundant protein biomarker used for diagnosing myocardial infarction, in human plasma by isotope-dilution LC-MS/MS analysis.



Similar content being viewed by others
References
Bunk DM, Dalluge JJ, Welch MJ. Heterogeneity in human cardiac troponin I standards. Anal Biochem. 2000;284(2):191–200.
Panteghini M, Bunk DM, Christenson RH, Katrukha A, Porter RA, Schimmel H, et al. Standardization of troponin I measurements: an update. Clin Chem Lab Med. 2008;46(11):1501–6.
Panteghini M. Assay-related issues in the measurement of cardiac troponins. Clin Chim Acta. 2009;402(1–2):88–93.
Panteghini M, Pagani F, Yeo KTJ, Apple FS, Christenson RH, Dati F, et al. Evaluation of imprecision for cardiac troponin assays at low-range concentrations. Clin Chem. 2004;50(2):327–32.
Bunk DM, Welch MJ. Characterization of a new certified reference material for human cardiac troponin I. Clin Chem. 2006;52(2):212–9.
Tate JR, Bunk DM, Christenson RH, Katrukha A, Noble JE, Porter RA, et al. Standardisation of cardiac troponin I measurement: past and present. Pathology. 2010;42(5):402–8.
Christenson RH, Duh SH, Apple FS, Bodor GS, Bunk DM, Dalluge J, et al. Standardization of cardiac troponin I assays: round robin of ten candidate reference materials. Clin Chem. 2001;47(3):431–7.
Wu AHB, Christenson RH. Analytical and assay issues for use of cardiac troponin testing for risk stratification in primary care. Clin Biochem. 2013;46(12):969–78.
Noble JE, Bunk DM, Christenson RH, Cole KD, He HJ, Katrukha AG, et al. Development of a candidate secondary reference procedure (immunoassay based measurement procedure of higher metrological order) for cardiac troponin I: I. antibody characterization and preliminary validation. Clin Chem Lab Med. 2010;48(11):1603–10.
Christenson RH, Duh SH, Apple FS, Bodor GS, Bunk DM, Panteghini M, et al. Toward standardization of cardiac troponin I measurements part II: assessing commutability of candidate reference materials and harmonization of cardiac troponin I assays. Clin Chem. 2006;52(9):1685–92.
Tate JR, Bunk DM, Christenson RH, Barth JH, Katrukha A, Noble JE, et al. Evaluation of standardization capability of current cardiac troponin I assays by a correlation study: results of an IFCC pilot project. Clin Chem Lab Med. 2015;53(5):677–90.
Villanueva J, Carrascal M, Abian J. Isotope dilution mass spectrometry for absolute quantification in proteomics: concepts and strategies. J Proteome. 2014;96:184–99.
Hoofnagle AN, Roth MY. Improving the measurement of serum thyroglobulin with mass spectrometry. J Clin Endocrinol Metab. 2013;98(4):1343–52.
Lange V, Picotti P, Domon B, Aebersold R. Selected reaction monitoring for quantitative proteomics: a tutorial. Mol Syst Biol. 2008;4:1–14.
Schneck NA, Lowenthal M, Phinney K, Lee SB. Current trends in magnetic particle enrichment for mass spectrometry-based analysis of cardiovascular protein biomarkers. Nanomedicine. 2015;10(3):433–46.
Kilpatrick EL, Bunk DM. Reference measurement procedure development for C-reactive protein in human serum. Anal Chem. 2009;81(20):8610–6.
Meng ZJ, Veenstra TD. Targeted mass spectrometry approaches for protein biomarker verification. J Proteome. 2011;74(12):2650–9.
Bunk DM. Reference materials and reference measurement procedures: an overview from a national metrology institute. Clin Biochem Rev. 2007;28(4):131–7.
Panteghini M. Standardization of cardiac troponin I measurements: the way forward? Clin Chem. 2005;51(9):1594–7.
Picard G, Lebert D, Louwagie M, Adrait A, Huillet C, Vandenesch F, et al. PSAQ (TM) standards for accurate MS-based quantification of proteins: from the concept to biomedical applications. J Mass Spectrom. 2012;47(10):1353–63.
Huillet C, Adrait A, Lebert D, Picard G, Trauchessec M, Louwagie M, et al. Accurate quantification of cardiovascular biomarkers in serum using protein standard absolute quantification (PSAQ (TM)) and selected reaction monitoring. Mol Cell Proteomics. 2012;11(2):12.
Keshishian H, Addona T, Burgess M, Mani DR, Shi X, Kuhn E, et al. Quantification of cardiovascular biomarkers in patient plasma by targeted mass spectrometry and stable isotope dilution. Mol Cell Proteomics. 2009;8(10):2339–49.
Keshishian H, Addona T, Burgess M, Kuhn E, Carr SA. Quantitative, multiplexed assays for low abundance proteins in plasma by targeted mass spectrometry and stable isotope dilution. Mol Cell Proteomics. 2007;6(12):2212–29.
Kuhn E, Addona T, Keshishian H, Burgess M, Mani DR, Lee RT, et al. Developing multiplexed assays for troponin I and interleukin-33 in plasma by peptide immunoaffinity enrichment and targeted mass spectrometry. Clin Chem. 2009;55(6):1108–17.
Zhao C, Trudeau B, Xie H, Prostko J, Fishpaugh J, Ramsay C. Epitope mapping and targeted quantitation of the cardiac biomarker troponin by SID-MRM mass spectrometry. Proteomics. 2014;14(11):1311–21.
Lowenthal MS, Liang YX, Phinney KW, Stein SE. Quantitative bottom-up proteomics depends on digestion conditions. Anal Chem. 2014;86(1):551–8.
Lowenthal MS, Gasca-Aragon H, Schiel JE, Dodder NG, Bunk DM. A quantitative LC-MS/MS method for comparative analysis of capture-antibody affinity toward protein antigens. J Chromatogr B-Analyt Technol Biomed Life Sci. 2011;879(26):2726–32.
Schneck NA, Phinney KW, Lee SB, Lowenthal MS. Quantification of antibody coupled to magnetic particles by targeted mass spectrometry. Anal Bioanal Chem. 2016;408(29):8325–32.
Rogstad SM, Sorkina T, Sorkin A, Wu CC. Improved precision of proteomic measurements in immunoprecipitation based purifications using relative quantitation. Anal Chem. 2013;85(9):4301–6.
Agewall S, Giannitsis E, Jernberg T, Katus H. Troponin elevation in coronary vs. non-coronary disease. Eur Heart J. 2011;32(4):404–11B.
Berna MJ, Zhen YJ, Watson DE, Hale JE, Ackermann BL. Strategic use of immunoprecipitation and LC/MS/MS for trace-level protein quantification: myosin light chain 1, a biomarker of cardiac necrosis. Anal Chem. 2007;79(11):4199–205.
Mani V, Chikkaveeraiah BV, Rusling JF. Magnetic particles in ultrasensitive biomarker protein measurements for cancer detection and monitoring. Expert Opin Med Diagn. 2011;5(5):381–91.
Peter JF, Otto AM. Magnetic particles as powerful purification tool for high sensitive mass spectrometric screening procedures. Proteomics. 2010;10(4):628–33.
Safarik I, Safarikova M. Magnetic techniques for the isolation and purification of proteins and peptides. Biomagn Res Technol. 2004;2(1):7.
Whiteaker JR, Zhao L, Zhang HY, Feng LC, Piening BD, Anderson L, et al. Antibody-based enrichment of peptides for mass-spectrometry-based quantification on magnetic beads of serum biomarkers. Anal Biochem. 2007;362(1):44–54.
Beasley-Green A, Burris NM, Bunk DM, Phinney KW. Multiplexed LC-MS/MS assay for urine albumin. J Proteome Res. 2014;13(9):3930–9.
He HJ, Lowenthal MS, Cole KD, Bunk D, Wang LL. An immunoprecipitation coupled with fluorescent western blot analysis for the characterization of a model secondary serum cardiac troponin I reference material. Clin Chim Acta. 2011;412(1–2):107–11.
Apple FS. COUNTERPOINT - Standardization of cardiac troponin I assays will not occur in my lifetime. Clin Chem. 2012;58(1):169–71.
Katrukha AG, Bereznikova AV, Filatov VL, Esakova TV, Kolosova OV, Pettersson K, et al. Degradation of cardiac troponin I: implication for reliable immunodetection. Clin Chem. 1998;44(12):2433–40.
Labugger R, Organ L, Collier C, Atar D, Van Eyk JE. Extensive troponin I and T modification detected in serum from patients with acute myocardial infarction. Circulation. 2000;102(11):1221–6.
Acknowledgements
The authors thank Dr. Andrew Hoofnagle from the University of Washington for providing clinical samples and assistance, and Dr. Eric Kilpatrick from the National Institute of Standards and Technology for his logistical help receiving the patient plasma samples and for his scientific discussions. We also acknowledge the support of the Professional Research Experience Program (PREP) through the University of Maryland, College Park and the National Institute of Standards and Technology.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Patient plasma samples were obtained as de-identified, residual clinical samples from the University of Washington. All work with human derived samples was reviewed and approved by the NIST Human Subjects Protection Office.
Conflict of interest
The authors declare that they have no conflicts of interest.
Additional information
Disclaimer
Certain commercial equipment, instruments, and materials are identified in this paper to specify the experimental procedures and analytical methods adequately. In no case does such identification imply recommendation or endorsement by the National Institute of Standards and Technology, nor does it imply that the materials or equipment are necessarily the best available for the purpose.
Electronic supplementary material
ESM 1
(PDF 543 kb)
Rights and permissions
About this article
Cite this article
Schneck, N.A., Phinney, K.W., Lee, S.B. et al. Quantification of cardiac troponin I in human plasma by immunoaffinity enrichment and targeted mass spectrometry. Anal Bioanal Chem 410, 2805–2813 (2018). https://doi.org/10.1007/s00216-018-0960-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0960-7