Abstract
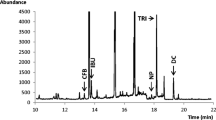
The present study explores the potential of 10-day-old zebrafish (Danio rerio) as a predictive blood-brain-barrier model using a set of 7 pharmaceutical agents. For this purpose, zebrafish were incubated with each of these 7 drugs separately via the route of immersion and the concentration reaching the brain was determined by applying a brain extraction procedure allowing isolation of the intact brain from the head of the zebrafish larvae. Sample analysis was performed utilizing capillary ultra-high performance liquid chromatography (cap-UHPLC) on a Pepmap RSLC C18 capillary column (150 mm × 300 μm, dp = 2 μm) coupled to a variable wavelength UV detector. Gradient separation was performed in 28 min at a flow rate of 5 μL/min and the optimal injection volume was determined to be 1 μL. The brain extraction procedure was established for the zebrafish strain TG898 exhibiting red fluorescence of the brain, allowing control of the integrity of the extracted parts. Quantitative experiments carried out on pooled samples of six zebrafish (n = 6) demonstrated the selective semipermeable nature of the blood-brain barrier after incubating the zebrafish at the maximum tolerated concentration for the investigated pharmaceuticals. The obtained brain-to-trunk ratios ranged between 0.3 for the most excluded compound and 1.2 for the pharmaceutical agent being most accumulated in the brain of the fish.

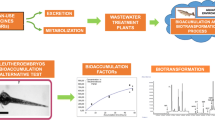
Workflow of brain extraction to study the uptake of pharmaceuticals in the brain of zebrafish larvae




Similar content being viewed by others
References
Engelhardt B. Development of the blood-brain barrier. Cell Tissue Res. 2003;314:119–29. https://doi.org/10.1007/s00441-003-0751-z.
Pardridge WM. The blood-brain barrier: bottleneck in brain drug development. NeuroRx. 2005;2:3–14. https://doi.org/10.1602/neurorx.2.1.3.
Reichel A. Addressing central nervous system (CNS) penetration in drug discovery: basics and implications of the evolving new concept. Chem Biodivers. 2009;6:2030–49. https://doi.org/10.1002/cbdv.200900103.
Hitchcock SA. Blood-brain barrier permeability considerations for CNS-targeted compound library design. Curr Opin Chem Biol. 2008;12:318–23. https://doi.org/10.1016/j.cbpa.2008.03.019.
Gabathuler R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol Dis. 2010;37:48–57. https://doi.org/10.1016/j.nbd.2009.07.028.
Nau R, Sorgel F, Eiffert H. Penetration of drugs through the blood-cerebrospinal fluid/blood-brain barrier for treatment of central nervous system infections. Clin Microbiol Rev. 2010;23:858–83. https://doi.org/10.1128/CMR.00007-10.
Banks WA. Characteristics of compounds that cross the blood-brain barrier. BMC Neurol. 2009;9:S3. https://doi.org/10.1186/1471-2377-9-S1-S3.
Pajouhesh H, Lenz GR. Medicinal chemical properties of successful central nervous system drugs. J Am Soc Exp Neurother. 2005;2:541–53. https://doi.org/10.1602/neurorx.2.4.541.
Schinkel AH, Wagenaar E, Mol CAAM, Van Deemter L. P-glycoprotein in the blood-brain barrier of mice influences the brain penetration and pharmacological activity of many drugs. J Clin Invest. 1996;97:2517–24. https://doi.org/10.1172/JCI118699.
Nielsen PA, Andersson O, Hansen SH, Simonsen KB, Andersson G. Models for predicting blood-brain barrier permeation. Drug Discov Today. 2011;16:472–5. https://doi.org/10.1016/j.drudis.2011.04.004.
Mensch J, L LJ, Sanderson W, Melis A, Mackie C, Verreck G, et al. Application of PAMPA-models to predict BBB permeability including efflux ratio, plasma protein binding and physicochemical parameters. Int J Pharm. 2010;395:182–97. https://doi.org/10.1016/j.ijpharm.2010.05.037.
Di L, Kerns EH, Fan K, McConnell OJ, Carter GT. High throughput artificial membrane permeability assay for blood-brain barrier. Eur J Med Chem. 2003;38:223–32.
Syvänen S, Lindhe Ö, Palner M, Lindhe O, Kornum BR, Rahman O, et al. Species differences in blood-brain barrier transport of three PET radioligands with emphasis on P-glycoprotein transport. Drug Metab Dispos. 2008;37:635–43. https://doi.org/10.1124/dmd.108.024745.
Lundquist S, Renftel M, Brillault J, Fenart L, Cecchelli R, Dehouck MP. Prediction of drug transport through the blood-brain barrier in vivo: a comparison between two in vitro cell models. Pharm Res. 2002;19:976–81.
Roux F, Couraud P-O. Rat brain endothelial cell lines for the study of blood-brain barrier permeability and transport functions. Cell Mol Neurobiol. 2005;25:41–58. https://doi.org/10.1007/s10571-004-1376-9.
Andersson O, Hansen SH, Hellman K, Olsen LR, Andersson G, Badolo L, et al. The grasshopper: a novel model for assessing vertebrate brain uptake. J Pharmacol Exp Ther. 2013;346:211–8. https://doi.org/10.1124/jpet.113.205476.
DeSalvo MK, Mayer N, Mayer F, Bainton RJ. Physiologic and anatomic characterization of the brain surface glia barrier of drosophila. Glia. 2011;59:1322–40. https://doi.org/10.1002/glia.21147.PHYSIOLOGIC.
Howe K, Clark MD, Torroja CF, Torrance J, Berthelot C, Muffato M, et al. The zebrafish reference genome sequence and its relationship to the human genome. Nature. 2013;496:498–503. https://doi.org/10.1038/nature12111.
Barros TP, Alderton WK, Reynolds HM, Roach AG, Berghmans S. Zebrafish: an emerging technology for in vivo pharmacological assessment to identify potential safety liabilities in early drug discovery. Br J Pharmacol. 2008;154:1400–13. https://doi.org/10.1038/bjp.2008.249.
Fleming A, Diekmann H, Goldsmith P. Functional characterisation of the maturation of the blood-brain barrier in larval zebrafish. PLoS One. 2013;8:1–12. https://doi.org/10.1371/journal.pone.0077548.
Escher BI, Ashauer R, Dyer S, Hermens JLM, Lee JH, Leslie HA, et al. Crucial role of mechanisms and modes of toxic action for understanding tissue residue toxicity and internal effect concentrations of organic chemicals. Integr Environ Assess Manag. 2011;7:28–49. https://doi.org/10.1002/ieam.100.
Tufi S, Leonards P, Lamoree M, De Boer J, Legler J, Legradi J. Changes in neurotransmitter profiles during early zebrafish (Danio rerio) development and after pesticide exposure. Environ Sci Technol. 2016;50:3222–30. https://doi.org/10.1021/acs.est.5b05665.
Berghmans S, Butler P, Goldsmith P, Waldron G, Gardner I, Golder Z, et al. Zebrafish based assays for the assessment of cardiac, visual and gut function—potential safety screens for early drug discovery. J Pharmacol Toxicol Methods. 2008;58:59–68. https://doi.org/10.1016/j.vascn.2008.05.130.
Beker van Woudenberg A, Snel C, Rijkmans E, De Groot D, Bouma M, Hermsen S, et al. Zebrafish embryotoxicity test for developmental (neuro)toxicity: demo case of an integrated screening approach system using anti-epileptic drugs. Reprod Toxicol. 2014;49:101–16. https://doi.org/10.1016/j.reprotox.2014.07.082.
Jones HS, Trollope HT, Hutchinson TH, Panter GH, Chipman JK. Metabolism of ibuprofen in zebrafish larvae. Xenobiotica. 2012;42:1069–75. https://doi.org/10.3109/00498254.2012.684410.
Kirla KT, Groh KJ, Steuer AE, Poetzsch M, Banote RK, Stadnicka-Michalak J, et al. Zebrafish larvae are insensitive to stimulation by cocaine: importance of exposure route and toxicokinetics. Toxicol Sci. 2016;154:183–93. https://doi.org/10.1093/toxsci/kfw156.
Chen F, Gong Z, Kelly BC. Rapid analysis of pharmaceuticals and personal care products in fish plasma micro-aliquots using liquid chromatography tandem mass spectrometry. J Chromatogr A. 2015;1383:104–11. https://doi.org/10.1016/j.chroma.2015.01.033.
Kühnert A, Vogs C, Altenburger R, Küster E. The internal concentration of organic substances in fish embryos—a toxicokinetic approach. Environ Toxicol Chem. 2013;32:1819–27. https://doi.org/10.1002/etc.2239.
Brox S, Ritter AP, Küster E, Reemtsma T. A quantitative HPLC-MS/MS method for studying internal concentrations and toxicokinetics of 34 polar analytes in zebrafish (Danio rerio) embryos. Anal Bioanal Chem. 2014;406:4831–40. https://doi.org/10.1007/s00216-014-7929-y.
Kantae V, Krekels EHJ, Ordas A, González O, van Wijk RC, Harms AC, et al. Pharmacokinetic modeling of paracetamol uptake and clearance in zebrafish larvae: expanding the allometric scale in vertebrates with five orders of magnitude. Zebrafish. 2016;13:504–10. https://doi.org/10.1089/zeb.2016.1313.
Kalueff AV, Stewart AM, Gerlai R, Court P. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol Sci. 2014;35:63–75. https://doi.org/10.1016/j.tips.2013.12.002.Zebrafish.
Jeong JY, Kwon HB, Ahn JC, Kang D, Kwon SH, Park JA, et al. Functional and developmental analysis of the blood-brain barrier in zebrafish. Brain Res Bull. 2008;75:619–28. https://doi.org/10.1016/j.brainresbull.2007.10.043.
Zhuo H, Jin H, Peng H, Huang H. Distribution, pharmacokinetics and primary metabolism model of tramadol in zebrafish. Mol Med Rep. 2016;14:5644–52. https://doi.org/10.3892/mmr.2016.5956.
Kim SS, Im SH, Yang JY, Lee Y-R, Kim GR, Chae JS, et al. Zebrafish as a screening model for testing the permeability of blood–brain barrier to small molecules. Zebrafish. 2017;14:322–30. https://doi.org/10.1089/zeb.2016.1392.
Kulkarni P, Chaudhari GH, Sripuram V, Banote RK, Kirla KT, Sultana R, et al. Oral dosing in adult zebrafish: proof-of-concept using pharmacokinetics and pharmacological evaluation of carbamazepine. Pharmacol Rep. 2014;66:179–83. https://doi.org/10.1016/j.pharep.2013.06.012.
Hrdina PD, Dubas TC. Brain distribution and kinetics of desipramine in the rat. Can J Physiol Pharrnacol. 1980;59:163–7.
Bicker J, Alves G, Fortuna A, Falcao A. Blood-brain barrier models and their relevance for a successful development of CNS drug delivery systems: a review. Eur J Pharm Biopharm. 2014;87:409–32. https://doi.org/10.1016/j.ejpb.2014.03.012.
Benya TJ, Wagner JG. Rapid equilibration of warfarin between rat tissue and plasma. J Pharmacokinet Biopharma. 1975;3:237-255.
DeVane CL, Boulton DW, Miller LF, Miller RL. Pharmacokinetics of trazodone and its major metabolite m-chlorophenylpiperazine in plasma and brain of rats. Int J Neuropsychopharmacol. 1999;2:17–23. https://doi.org/10.1017/S1461145799001303.
O’Byrne PM, Williams R, Walsh JJ, Gilmer JF. Part two: evaluation of N-methylbupropion as a potential bupropion prodrug. Pharmaceuticals. 2014;7:676–94. https://doi.org/10.3390/ph7060676.
Hendrickx S, Uğur DY, Yilmaz IT, Şener E, Van Schepdael A, Adams E, et al. A sensitive capillary LC-UV method for the simultaneous analysis of olanzapine, chlorpromazine and their FMO-mediated N-oxidation products in brain microdialysates. Talanta. 2017;162:268–77. https://doi.org/10.1016/j.talanta.2016.09.053.
Kislyuk S, Kroonen J, Adams E, Augustijns P, De Witte P, Cabooter D. Development of a sensitive and quantitative UHPLC-MS/MS method to study the whole-body uptake of pharmaceuticals in zebrafish. Talanta. 2017;174:780–8. https://doi.org/10.1016/j.talanta.2017.06.075.
EMA. Guideline on bioanalytical method validation. EMEA, Comm Med Prod Hum Use. 2012;44:1–23.
Lee M, Hong S, Kim N, Shin KS, Kang SJ. Tricyclic antidepressants amitriptyline and desipramine induced neurotoxicity associated with Parkinson’s disease. Mol Cells. 2015;38:734–40. https://doi.org/10.14348/molcells.2015.0131.
Acknowledgements
ARIADME, a European FP7 ITN Community’s Seventh Framework Program under Grant Agreement No. 607517, is acknowledged for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All animal experiments were approved by the Animal Ethical Committee of the University of Leuven (approval no. P007/2016) and conducted in accordance with the Belgian and European laws, guidelines, and policies for animal experimentation, housing, and care (Belgian Royal Decree of 29 May 2013 and European Directive 2010/63/EU on the protection of animals used for scientific purposes of 20 October 2010).
Conflict of interest
The authors declare that they have no conflict of interest.
Electronic supplementary material
ESM 1
(PDF 994 kb)
Rights and permissions
About this article
Cite this article
Kislyuk, S., Van den Bosch, W., Adams, E. et al. Development of a sensitive and quantitative capillary LC-UV method to study the uptake of pharmaceuticals in zebrafish brain. Anal Bioanal Chem 410, 2751–2764 (2018). https://doi.org/10.1007/s00216-018-0955-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0955-4