Abstract
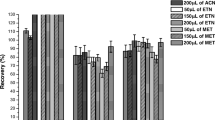
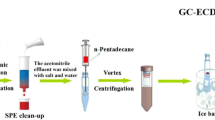
This study developed a new effervescence-assisted switchable fatty acid-based microextraction combined with solidification of a floating organic-droplet (EA-SFAM-SFO) for simple and rapid determination of fluoroquinolones and tetracyclines in seawater, sediment, and seafood. Five medium-chain fatty acids (pentanoic acid, hexanoic acid, heptanoic acid, octanoic acid, and nonanoic acid) were tested as an extraction solvent, given their ability to change between hydrophobic and hydrophilic forms by pH adjustment. As nonanoic acid had the highest extraction recovery (>92%) for the six antibiotics and the ability to transform from liquid to a solidified floating state at low temperature, it was selected as the optimum extraction solvent. The prominent advantages of the newly developed method are: (1) reaction between the procedures salt and fatty acid changed extraction solvent from the hydrophobic to hydrophilic state; (2) bubbling with CO2 greatly increased the contact area between fatty acid and analytes resulting in improved extraction recovery; and (3) solidification of the fatty acid at a low temperature provided good separation and avoided the use of specialized equipment. Single-factor screening and optimization of the main factors were conducted using Plackett-Burman design and central composite design, respectively. The main parameters were optimized as follows: 258 μL fatty acid, 406 μL H2SO4 (98%), 3.9 min vortex time, and 354 μL Na2CO3 (2 mol L-1). Under optimized conditions, limits of detection were 0.007–0.113 μg L-1 or μg kg-1 and extraction recoveries were 82.2%–116.7% for six fluoroquinolone and tetracycline antibiotics in seawater, sediments, and seafood. The newly developed method combines the advantages of effervescence-assisted dispersion, hydrophobic/hydrophilic switchable solvent, and liquid/solid transition induced by low temperature. Overall, the new method is simple, quick, and environment-friendly with low detection limits and high recoveries. Thus, the newly developed method has excellent prospects for sample pretreatment and analysis of antibiotics in marine environmental and food samples.

ᅟ





Similar content being viewed by others
References
Gao S, Jin H, You J, Ding Y, Zhang N, Wang Y, et al. Ionic liquid-based homogeneous liquid–liquid microextraction for the determination of antibiotics in milk by high-performance liquid chromatography. J Chromatogr A. 2011;1218:7254–63.
Toelgyesi A, Toelgyesi L, Bekesi K, Sharma VK, Fekete J. Determination of tetracyclines in pig and other meat samples using liquid chromatography coupled with diode array and tandem mass spectrometric detectors. Meat Sci. 2014;96:1332–9.
Ahmed MJ. Adsorption of quinolone, tetracycline, and penicillin antibiotics from aqueous solution using activated carbons: review. Environ Toxicol Pharmacol. 2017;50:1–10.
Nasr M, Yazdanbakhsh A. Study on wastewater treatment systems in hospitals of Iran. Iran. J Environ Health. 2008;5:211–5.
Karthikeyan K, Meyer M. Occurrence of antibiotics in wastewater treatment facilities in Wisconsin, USA. Sci Total Environ. 2006;361:196–207.
Zhou L, Ying G, Liu S, Zhao J, Yang B, Chen Z, et al. Occurrence and fate of eleven classes of antibiotics in two typical wastewater treatment plants in South China. Sci Total Environ. 2013;452:365–76.
Davis J, Truman C, Kim S, Carlson K. Antibiotic transport via runoff and soil loss. J Environ Qual. 2006;35:2250–60.
Na G, Fang X, Cai Y, Ge L, Zong H, Yuan X, et al. Occurrence, distribution, and bioaccumulation of antibiotics in coastal environment of Dalian, China. Mar Pollut Bull. 2013;69:233–7.
Boxall A, Kolpin D, Halling-Sorensen B, Tolls J. Are veterinary medicines causing environmental risks? Environ Sci Technol. 2003;37:286–94.
Na G, Gu J, Ge L, Zhang P, Wang Z, Liu C, et al. Detection of 36 antibiotics in coastal waters using high performance liquid chromatography-tandem mass spectrometry. Chin J Oceanol Limnol. 2011;29:1093–102.
Liu X, Liu Y, Xu J, Ren K, Meng X. Tracking aquaculture-derived fluoroquinolones in a mangrove wetland, South China. Environ Pollut. 2016;219:916–23.
Cohen S, Yan W, Levy S. A multidrug resistance regulatory chromosomal locus is widespread among enteric bacteria. J Infect Dis. 1993;168:484–8.
Pruden A, Pei R, Storteboom H, Carlson K. Antibiotic resistance genes as emerging contaminants: studies in northern Colorado. Environ Sci Technol. 2006;40:7445–50.
Schwaiger J, Ferling H, Mallow U, Wintermayr H, Negele R. Toxic effects of the non-steroidal anti-inflammatory drug diclofenac. Part I: Histopathological alterations and bioaccumulation in rainbow trout. Aquat Toxicol. 2004;68:141–50.
Mimeault C, Woodhouse A, Miao X, Metcalfe C, Moon T, Trudeau V. The human lipid regulator, gemfibrozil, bioconcentrates and reduces testosterone in the goldfish, Carassius auratus. Aquat Toxicol. 2005;73:44–54.
Rampal S, Kaur R, Sethi R, Singh O, Sood N. Ofloxacin-associated retinopathy in rabbits: role of oxidative stress. Hum Exp Toxicol. 2008;27:409–15.
Zhao B, Chignell CF, Rammal M, Smith F, Hamilton MG, Andley UP, et al. Detection and prevention of ocular phototoxicity of ciprofloxacin and other fluoroquinolone antibiotics. Photochem Photobiol. 2010;86:798–805.
Batt A, Bruce I, Aga D. Evaluating the vulnerability of surface waters to antibiotic contamination from varying wastewater treatment plant discharges. Environ Pollut. 2006;142:295–302.
Białk-Bielińska A, Kumirska J, Borecka M, Caban M, Paszkiewicz M, Pazdro K, et al. Selected analytical challenges in the determination of pharmaceuticals in drinking/marine waters and soil/sediment samples. J Pharmaceut Biomed. 2016;121:271–96.
Kuban P, Bocek P. Direct coupling of supported liquid membranes to capillary electrophoresis for analysis of complex samples: a tutorial. Anal Chim Acta. 2013;787:10–23.
Pochivalov A, Timofeeva I, Vakh C, Bulatov A. Switchable hydrophilicity solvent membrane-based microextraction: HPLC-FLD determination of fluoroquinolones in shrimps. Anal Chim Acta. 2017;976:35–44.
Xu W, Zhang G, Zou S, Li X, Liu Y. Determination of selected antibiotics in the Victoria Harbor and the Pearl River, South China, using high-performance liquid chromatography-electrospray ionization tandem mass spectrometry. Environ Pollut. 2007;145:672–9.
Siedlewicz G, Borecka M, Białk-Bielińska A, Sikora K, Stepnowski P, Pazdro K. Determination of antibiotic residues in southern Baltic Sea sediments using tandem solid-phase extraction and liquid chromatography coupled with tandem mass spectrometry. Oceanologia. 2016;58:221–34.
Rezaee M, Assadi Y, Hosseini MRM, Aghaee E, Ahmadi F, Berijani S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J Chromatogr A. 2006;1116:1–9.
Kocúrová L, Balogh I, Šandrejová J, Andruch V. Recent advances in dispersive liquid–liquid microextraction using organic solvents lighter than water: a review. Microchem J. 2012;102:11–7.
Saraji M, Boroujeni M. Recent developments in dispersive liquid–liquid microextraction. Anal Bioanal Chem. 2014;406:2027–66.
Saleh A, Yamini Y, Faraji M, Rezaee M, Ghambarian M. Ultrasound-assisted emulsification microextraction method based on applying low density organic solvents followed by gas chromatography analysis for the determination of polycyclic aromatic hydrocarbons in water samples. J Chromatogr A. 2009;1216:6673–9.
Farajzadeh M, Mohebbi A, Feriduni B. Development of continuous dispersive liquid–liquid microextraction performed in home-made device for extraction and preconcentration of aryloxyphenoxy-propionate herbicides from aqueous samples followed by gas chromatography-flame ionization detection. Anal Chim Acta. 2016;920:1–9.
Leong M, Huang S. Dispersive liquid–liquid microextraction method based on solidification of floating organic drop combined with gas chromatography with electron-capture or mass spectrometry detection. J Chromatogr A. 2008;1211:8–12.
Wang Y, Zhu C, Zou X, Huang L, Yan D. Solvent demulsification-dispersive liquid–liquid microextraction based on solidification of floating organic drop coupled with gas chromatography-mass spectrometry for simultaneous determination of organochlorine pesticides in aqueous samples. Chin J Chromatogr. 2013;31:1076–80.
Wang J, Zhang H, Chen J, Zhang L. Dispersive liquid–liquid microextraction based on solidification of floating organic droplets combined with high performance liquid chromatography-tandem mass spectrometry for determination of benzotriazole ultraviolet stabilizers in seawater. Chin J Chromatogr. 2014;32:913–8.
Cistola D, Hamilton J, Jackson D, Small D. Ionization and phase behavior of fatty acids in water: application of the Gibbs phase rule. Biochemistry. 1988;27:1881–8.
Igarashi S, Yotsuyanagi T. Homogeneous liquid–liquid extraction by pH-dependent phase separation with a fluorocarbon ionic surfactant and its application to the preconcentration of porphyrin compounds. Microchim Acta. 1992;106:37–44.
Shih H, Shu T, Ponnusamy V, Jen J. A novel fatty acid-based in-tube dispersive liquid–liquid microextraction technique for the rapid determination of nonylphenol and 4-tert-octylphenol in aqueous samples using high-performance liquid chromatography-ultraviolet detection. Anal Chim Acta. 2015;854:70–7.
Vakh C, Pochivalov A, Andruch V, Moskvin L, Bulatov A. A fully automated effervescence-assisted switchable solvent-based liquid phase microextraction procedure: liquid chromatographic determination of ofloxacin in human urine samples. Anal Chim Acta. 2016;907:54–9.
Ma L, Wang L, Tang J, Yang Z. Optimization of arsenic extraction in rice samples by Plackett-Burman design and response surface methodology. Food Chem. 2016;204:283–8.
Vander H, Nijhuis A, Smeyers-Verbeke J, Vandeginste B, Massart D. Guidance for robustness/ruggedness tests in method validation. J Pharmaceut Biomed. 2001;24:723–53.
Heyden Y, Luypaert K, Hartmann C, Massart L, Hoogmartens J, Beer D. Ruggedness tests on the high-performance liquid chromatography assay of the United States Pharmacopeia xxii for tetracycline hydrochloride. A comparison of experimental designs and statistical interpretations. Anal Chim Acta. 1995;312:245–62.
Borges P, Tavares E, Guimarães I, Rocha R, Araujo A, Nunes E, et al. Obtaining a protocol for extraction of phenolics from Açaífruit pulp through Plackett-Burman design and response surface methodology. Food Chem. 2016;210:189–99.
Fabre H, Mesplet N. Robustness testing for a capillary electrophoresis method using the “short-end injection” technique. J Chromatogr A. 2000;897:329–38.
Wang H, Gao M, Xu Y, Wang W, Lian Z, Dahlgren R, et al. A phase separation method for analyses of fluoroquinolones in meats based on ultrasound-assisted salt-induced liquid–liquid microextraction and a new integrated device. Meat Sci. 2015;106:61–8.
Zheng P. Solubility of sodium carbonate. Soda Ind. 1984;2:53–5. in Chinese
Yan X. The solubility of phenol and sodium bicarbonate. Chem Educ. 2003;5:42. (in Chinese)
Sereshti H, Heravi YE, Samadi S. Optimized ultrasound-assisted emulsification microextraction for simultaneous trace multielement determination of heavy metals in real water samples by ICP-OES. Talanta. 2012;97:235–41.
Andersen W, Roybal J, Gonzales S, Turnipseed S, Pfenning A, Kuck L. Determination of tetracycline residues in shrimp and whole milk using liquid chromatography with ultraviolet detection and residue confirmation by mass spectrometry. Anal Chim Acta. 2005;529:145–50.
Li W, Shi Y, Gao L, Liu J, Cai Y. Investigation of antibiotics in mollusks from coastal waters in the Bohai Sea of China. Environ Pollut. 2012;162:56–62.
Liu Y, Yang H, Yang S, Hu Q, Cheng H. High-performance liquid chromatography using pressurized liquid extraction for the determination of seven tetracyclines in egg, fish, and shrimp. J Chromatogr B. 2013;917/918:11–7.
Sun X, Wang J, Li Y, Yang J, Jin J, Shah S, et al. Novel dummy molecularly imprinted polymers for matrix solid-phase dispersion extraction of eight fluoroquinolones from fish samples. J Chromatogr A. 2014;1359:1–7.
Jiang J, Zhang H, Wang Z. Multiresidue determination of sarafloxacin, difloxacin, norfloxacin, and pefloxacin in fish using an enzyme-linked immunosorbent assay. Procedia Environ Sci. 2011;8:301–6.
Samanidou V, Evaggelopoulou E, Trötzmüller M, Guo X, Lankmayr E. Multi-residue determination of seven quinolones antibiotics in gilthead seabream using liquid chromatography-tandem mass spectrometry. J Chromatogr A. 2008;1203:115–23.
Chen B, Han J, Wang Y, Sheng C, Liu Y, Zhang G, et al. Separation, enrichment, and determination of ciprofloxacin using thermoseparating polymer aqueous two-phase system combined with high performance liquid chromatography in milk, egg, and shrimp samples. Food Chem. 2014;148:105–11.
Ranjbari E, Hadjmohammadi M, Kiekens F, Wael K. Mixed hemi/ad-micelle sodium dodecyl sulfate-coated magnetic iron oxide nanoparticles for the efficient removal and trace determination of rhodamine-b and rhodamine-6g. Anal Chem. 2015;87:7894–901.
Acknowledgements
This work was jointly supported by the National Natural Science Foundation of China (41676100 and 21577107).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Ming Gao declares that he has no conflict of interest. Jun Wang declares that he has no conflict of interest. Xiukai Song declares that he has no conflict of interest. Xin He declares that he has no conflict of interest. Randy A. Dahlgren declares that he has no conflict of interest. Zhenzhong Zhang declares that he has no conflict of interest. Shaoguo Ru has received research grants from the National Natural Science Foundation of China (41676100). Xuedong Wang has received research grants from the National Natural Science Foundation of China (21577107).
Electronic supplementary material
ESM 1
(PDF 536 kb)
Rights and permissions
About this article
Cite this article
Gao, M., Wang, J., Song, X. et al. An effervescence-assisted switchable fatty acid-based microextraction with solidification of floating organic droplet for determination of fluoroquinolones and tetracyclines in seawater, sediment, and seafood. Anal Bioanal Chem 410, 2671–2687 (2018). https://doi.org/10.1007/s00216-018-0942-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-018-0942-9