Abstract
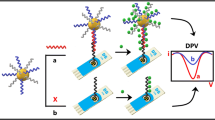
A new analytical method has been developed to detect three kinds of DNA simultaneously based on magnetic beads and color-encoded plasmonic nanocrystals. Magnetic beads modified with capture DNA are employed to collect the specific target DNA, and color-encoded plasmonic nanocrystals are applied to signal the target through DNA hybridization. As a proof of concept, three types of representative metal nanocrystals of gold nanoparticle (AuNP), gold nanorod (AuNR), and gold/silver nanoparticle (Au/AgNP) were employed to signal three dissimilar virus-related protective antigen genes, Ebola virus (EV), Variola virus (VV), and Bacillus anthracis (BA), respectively. Detection limits of 0.5–3 fM were obtained showing the high sensitivity for DNA detection. The microscopic discrimination of the encoded nanoparticles allows simple, rapid, accurate, and cost-effective detection of multiple DNA molecules, indicative of the potential in practical applications.

Development of a novel digital triplex DNA assay based on single-countable color-encoded plasmonic nanocrystals








Similar content being viewed by others
References
Lockhart DJ, Winzeler EA, Lockhart DJ, Winzeleer EA. Genomics, gene expression and DNA arrays. Nature. 2000;405(6788):827–36.
Qavi AJ, Bailey RC. Multiplexed detection and label-free quantitation of microRNAs using arrays of silicon photonic microring resonators. Angew Chem. 2010;49(27):4712–5.
Zhang M, Liu YQ, Yu CY, Yin BC, Ye BC. Multiplexed detection of microRNAs by tuning DNA-scaffolded silver nanoclusters. Analyst. 2013;138(17):4812–7.
Zheng W, He L. Label-free, real-time multiplexed DNA detection using fluorescent conjugated polymers. J Am Chem Soc. 2009;131(10):3432–3.
Yang Y. Sensitive fluorescent sensing for DNA assay. TrAC Trends Anal Chem. 2010;29(9):980–1003.
Cao YWC, Jin R, Mirkin CA. Nanoparticles with Raman spectroscopic fingerprints for DNA and RNA detection. Science. 2002;297(5586):1536–40.
Zhang Z, Wen Y, Ma Y, Luo J, Jiang L, Song Y. Mixed DNA-functionalized nanoparticle probes for surface-enhanced Raman scattering-based multiplex DNA detection. Chem Commun. 2011;47(26):7407–9.
Zhang CY, Hu J. Single quantum dot-based nanosensor for multiple DNA detection. Anal Chem. 2010;82(5):1921–7.
Li L, Wang X, Zhang X, Wang J, Jin W. Single-cell multiple gene expression analysis based on single-molecule-detection microarray assay for multi-DNA determination. Anal Chim Acta. 2015;854C:122–8.
Mancuso M, Jiang L, Cesarman E, Erickson D. Multiplexed colorimetric detection of Kaposi’s sarcoma associated herpes virus and Bartonella DNA using gold and silver nanoparticles. Nano. 2013;5(4):1678–86.
Wang C, Irudayaraj J. Gold nanorod probes for the detection of multiple pathogens. Small. 2008;4(12):2204–8.
Guo Y, Wang Z, Qu W, Shao H, Jiang X. Colorimetric detection of mercury, lead and copper ions simultaneously using protein-functionalized gold nanoparticles. Biosens Bioelectron. 2011;26(10):4064–9.
Han G, Zhang S, Xing Z, Zhang X. Absolute and relative quantification of multiplex DNA assays based on an elemental labeling strategy. Angew Chem Int Ed. 2013;52(5):1466–71.
Zhang S, Han G, Xing Z, Zhang S, Zhang X. Multiplex DNA assay based on nanoparticle probes by single particle inductively coupled plasma mass spectrometry. Anal Chem. 2014;86(7):3541–7.
Sepúlveda B, Angelomé PC, Lechuga LM, Liz-Marzán LM. LSPR-based nanobiosensors. Nano Today. 2009;4(3):244–51.
Khlebtsov NG, Dykman LA. Optical properties and biomedical applications of plasmonic nanoparticles. J Quant Spectrosc Radiat Transf. 2010;111(111):1–35.
Sönnichsen C, Alivisatos AP. Gold nanorods as novel nonbleaching plasmon-based orientation sensors for polarized single-particle microscopy. Nano Lett. 2005;5(2):301–4.
Hao J, Xiong B, Cheng XD, He Y, Yeung ES. High-throughput sulfide sensing with colorimetric analysis of single Au-Ag core-shell nanoparticles. Anal Chem. 2014;86(10):4663–7.
Saha K, Agasti SS, Kim C, Li X, Rotello VM. Gold nanoparticles in chemical and biological sensing. Chem Rev. 2012;112(5):2739–79.
Dreaden EC, Alkilany AM, Huang X, Murphy CJ, Elsayed MA. The golden age: gold nanoparticles for biomedicine. Chem Soc Rev. 2011;41(7):2740–79.
Tang D, Yu Y, Niessner R, Miró M, Knopp D. Magnetic bead-based fluorescence immunoassay for aflatoxin B1 in food using biofunctionalized rhodamine B-doped silica nanoparticles. Analyst. 2010;135(10):2661–7.
Xiang DS, Zeng GP, He Z. Magnetic microparticle-based multiplexed DNA detection with biobarcoded quantum dot probes. Biosens Bioelectron. 2011;26(11):4405–10.
Basilevsky MV, Shamov AG. Topical review: Functionalisation of magnetic nanoparticles for applications in biomedicine. J Phys D Appl Phys. 2003;36(36):R198–R206(9).
Nakagawa T, Seino S, Yamamoto TA, Abe M. Biomedical applications of magnetic beads. Teion Kogaku. 2010;45(10):436–43.
Nam JM, Thaxton CS, Mirkin CA. Nanoparticle-based bio-bar codes for the ultrasensitive detection of proteins. Science. 2003;301(5641):1884–6.
Shad Thaxton C, Hill HD, Georganopoulou DG, Stoeva SI, Mirkin CA. A bio-bar-code assay based upon dithiothreitol-induced oligonucleotide release. Anal Chem. 2006;77(24):8174–8.
Duan J, Park K, Maccuspie RI, Vaia RA, Pachter R. Optical properties of rod like metallic nanostructures: insight from theory and experiment. J Phys Chem C. 2009;113(35):15524–32.
Ziegler C, Eychmüller A. Seeded growth synthesis of uniform gold nanoparticles with diameters of 15−300 nm. J Phys Chem C. 2011;115(11):4502–6.
Xiao L, Wei L, He Y, Yeung ES. Single molecule biosensing using color coded plasmon resonant metal nanoparticles. Anal Chem. 2010;82(14):6308–14.
Pekcevik IC, Poon LC, Wang MC, Gates BD. Tunable loading of single-stranded DNA on gold nanorods through the displacement of polyvinylpyrrolidone. Anal Chem. 2013;85(20):9960–7.
Cheng X, Dai D, Yuan Z, Peng L, He Y, Yeung ES. Color difference amplification between gold nanoparticles in colorimetric analysis with actively controlled multiband illumination. Anal Chem. 2014;86(15):7584–92.
Nath N, Chilkoti A. A colorimetric gold nanoparticle sensor to interrogate biomolecular interactions in real time on a surface. Anal Chem. 2002;74(3):504–9.
Mayer KM, Lee S, Liao H, Rostro BC, Fuentes A, Scully PT, et al. A label-free immunoassay based upon localized surface plasmon resonance of gold nanorods. ACS Nano. 2008;2(4):687–92.
Byun JY, Shin YB, Li T, Park JH, Kim DM, Choi DH, et al. The use of an engineered single chain variable fragment in a localized surface plasmon resonance method for analysis of the C-reactive protein. Chem Commun. 2013;49(82):9497–9.
Stoeva SI, Lee JS, Thaxton CS, Mirkin CA. Multiplexed DNA detection with biobarcoded nanoparticle probes. Angew Chem. 2006;118(20):3381–4.
Acknowledgements
This work was supported by the National Science Foundation of China (grants 21273126 and 21573124) and the Fundamental Research Program of Shenzhen (JCYJ20140509172959966, JCYJ20160317152359560). HT thanks the financial support from The Science Technology Innovation Commission of Shenzhen Municipality (GJHZ20160301163644983) and The Health and Family Planning Commission of Shenzhen Municipality (201601019).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Rights and permissions
About this article
Cite this article
Li, G., Zhu, L., He, Y. et al. Digital triplex DNA assay based on plasmonic nanocrystals. Anal Bioanal Chem 409, 3657–3666 (2017). https://doi.org/10.1007/s00216-017-0307-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-017-0307-9