Abstract
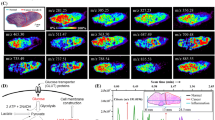
Imaging mass spectrometry is becoming a reference technique in the field of lipidomics, due to its ability to map the distribution of hundreds of species in a single run, along a tissue section. The next frontier is now achieving increasing resolution powers to offer cellular (or even sub-cellular) resolution. Thus, the new spectrometers are equipped with sophisticated optical systems to decrease the laser spot to <30 μm. Here, we demonstrate that by using the correct matrix (i.e., a matrix that maximizes ion detection and forms small crystals) and a careful preparation, it is possible to achieve resolutions of ∼5–10 μm, even with spectrometers equipped with non-optimal optics, which produces laser spots of 50 μm or even larger. As a proof of concept, we present images of distributions of lipids, both in positive and negative ion mode, over human colon endoscopic sections, recorded using 2-mercaptobenzothiazole for positive ion mode and 2,5-diaminonaphtalene for negative ion mode and an LTQ-Orbitrap XL, equipped with a matrix-assisted laser desorption ionization (MALDI) source that produces astigmatic laser spots.

Imaging mass spectrometry is becoming an invaluable technique to complement traditional histology, but still higher resolutions are required. Here we deal with such issue







Similar content being viewed by others
Abbreviations
- Cer:
-
Ceramide
- Cer-PE:
-
Ceramide phosphatidylethanolamine
- DAG:
-
Diacylglycerol
- DAG-O:
-
Diacylglycerol ether
- DAN:
-
2,5-diaminonaphtalene
- MBT:
-
2-mercaptobenzothiazole
- PA:
-
Glycerophosphatidic acid
- PC:
-
Glycerophophatidylcholine
- PE:
-
Glycerophosphatidyl ethanolamine
- PI:
-
Glycerophosphatidylinositol
- PS:
-
Glycerophosphatidylserine
- S/N:
-
Signal-to-noise ratio
- SFT:
-
Sulfatide
- SM:
-
Sphingomyelin
- TAG:
-
Triacylglycerol
References
Spengler B, Hubert M, Kaufmann R (1994) MALDI ion imaging and biological ion imaging with a new scanning UV-laser microprobe. Proceedings of the 42nd ASMS Conference on MassSpectrometry and Allied Topics
Stoeckli M, Farmer TB, Caprioli RM (1999) Automated mass spectrometry imaging with a matrix-assisted laser desorption ionization time-of-flight instrument. J Am Soc Mass Spectrom 10:67–71
Todd PJ, Schaaff TG, Chaurand P, Caprioli RM (2001) Organic ion imaging of biological tissue with secondary ion mass spectrometry and matrix-assisted laser desorption/ionization. J Mass Spectrom 36:355–369
Stoeckli M, Chaurand P, Hallahan DE, Caprioli RM (2001) Imaging mass spectrometry: a new technology for the analysis of protein expression in mammalian tissues. Nat Med 7:493–496
Fernandez JA, Ochoa B, Fresnedo O, Giralt MT, Rodriguez-Puertas R (2011) Matrix-assisted laser desorption ionization imaging mass spectrometry in lipidomics. Anal Bioanal Chem 401:29–51
Ellis S, Bruinen A, Heeren RA (2014) A critical evaluation of the current state-of-the-art in quantitative imaging mass spectrometry. Anal Bioanal Chem 406:1275–1289
Eriksson C, Masaki N, Yao I, Hayasaka T, Setou M (2013) MALDI imaging mass spectrometry—a mini review of methods and recent developments. Mass Spectrom 2:S0022–S0022
Kettling H, Vens-Cappell S, Soltwisch J, Pirkl A, Haier J, Müthing J, Dreisewerd K (2014) MALDI mass spectrometry imaging of bioactive lipids in mouse brain with a Synapt G2-S mass spectrometer operated at elevated pressure: improving the analytical sensitivity and the lateral resolution to ten micrometers. Anal Chem 86:7798–7805
Passarelli MK, Wang J, Mohammadi AS, Trouillon R, Gilmore I, Ewing AG (2014) Development of an organic lateral resolution test device for imaging mass spectrometry. Anal Chem 86:9473–4980. doi:10.1021/ac501228x
Anderson DG, Ablonczy Z, Koutalos Y, Spraggins J, Crouch R, Caprioli R, Schey K (2014) High resolution MALDI imaging mass spectrometry of retinal tissue lipids. J Am Soc Mass Spectrom 25:1394–1403
Freitas RA Jr (1999) Nanomedicine, volume I: basic capabilities. Landes Bioscience, Georgetown
Dreisewerd K (2014) Recent methodological advances in MALDI mass spectrometry. Anal Bioanal Chem 406:2261–2278
Korte A, Yandeau-Nelson M, Nikolau B, Lee Y (2015) Subcellular-level resolution MALDI-MS imaging of maize leaf metabolites by MALDI-linear ion trap-Orbitrap mass spectrometer. Anal Bioanal Chem 407:2301–2309
Murphy RC, Hankin JA, Barkley RM (2009) Imaging of lipid species by MALDI mass spectrometry. J Lipid Res 50:S317–S322
Spraggins J, Caprioli R (2011) High-speed MALDI-TOF imaging mass spectrometry: rapid ion image acquisition and considerations for next generation instrumentation. J Am Soc Mass Spectrom 22:1022–1031
Jurchen JC, Rubakhin SS, Sweedler JV (2005) MALDI-MS imaging of features smaller than the size of the laser beam. J Am Soc Mass Spectrom 16:1654–1659
Astigarraga E, Barreda-Gomez G, Lombardero L, Fresnedo O, Castano F, Giralt MT, Ochoa B, Rodriguez-Puertas R, Fernandez JA (2008) Profiling and imaging of lipids on brain and liver tissue by matrix-assisted laser desorption/ionization mass spectrometry using 2-mercaptobenzothiazole as a matrix. Anal Chem 80:9105–9114
Puolitaival SM, Burnum KE, Cornett DS, Caprioli RM (2008) Solvent-free matrix dry-coating for MALDI imaging of phospholipids. J Am Soc Mass Spectrom 19:882–886
Hankin JA, Barkley RM, Murphy RC (2007) Sublimation as a method of matrix application for mass spectrometric imaging. J Am Soc Mass Spectrom 18:1646–1652
Goodwin RJA, Scullion P, MacIntyre L, Watson DG, Pitt AR (2010) Use of a solvent-free dry matrix coating for quantitative matrix-assisted laser desorption ionization imaging of 4-bromophenyl-1,4-diazabicyclo(3.2.2)nonane-4-carboxylate in rat brain and quantitative analysis of the drug from laser microdissected tissue regions. Anal Chem 82:3868–3873
Trimpin S, Herath TN, Inutan ED, Wager-Miller J, Kowalski P, Claude E, Walker JM, Mackie K (2010) Automated solvent-free matrix deposition for tissue imaging by mass spectrometry. Anal Chem 82:359–367
Thomas A, Charbonneau JL, Fournaise E, Chaurand P (2012) Sublimation of new matrix candidates for high spatial resolution imaging mass spectrometry of lipids: enhanced information in both positive and negative polarities after 1,5-diaminonapthalene deposition. Anal Chem 84:2048–2054
Tempez A, Ugarov M, Egan T, Schultz JA, Novikov A, Della-Negra S, Lebeyec Y, Pautrat M, Caroff M, Smentkowski VS, Wang HJ, Jackson SN, Woods AS (2005) Matrix implanted laser desorption ionization (MILDI) combined with ion mobility-mass spectrometry for bio-surface analysis. J Proteome Res 4:540–545
Jackson S, Baldwin K, Muller L, Womack V, Schultz JA, Balaban C, Woods A (2014) Imaging of lipids in rat heart by MALDI-MS with silver nanoparticles. Anal Bioanal Chem 406:1377–1386
Fernández R, Lage S, Abad-García B, Barceló-Coblijn G, Terés S, López DH, Guardiola-Serrano F, Martín ML, Escribá PV, Fernández JA (2014) Analysis of the lipidome of xenografts using MALDI-IMS and UHPLC-ESI-QTOF. J Am Soc Mass Spectrom 25:1237–1246
Deininger SO, Ebert MP, Futterer A, Gerhard M, Rocken C (2008) MALDI imaging combined with hierarchical clustering as a new tool for the interpretation of complex human cancers. J Proteome Res 7:5230–5236
Xiong XC, Xu W, Eberlin LS, Wiseman JM, Fang X, Jiang Y, Huang ZJ, Zhang YK, Cooks RG, Ouyang Z (2012) Data processing for 3D mass spectrometry imaging. J Am Soc Mass Spectrom 23:1147–1156
Wold S, Esbensen K, Geladi P (1987) Principal component analysis. Chemom Intell Lab Syst 2:37–52
Arthur D, Vassilvitskii S (2007) k-means++: the advantages of careful seeding. Proceedings of the eighteenth annual ACM-SIAM symposium on discrete algorithms: 1027–1035
Strupat K, Kovtoun V, Bui H, Viner R, Stafford G, Horning S (2009) MALDI produced ions inspected with a linear ion trap-orbitrap hybrid mass analyzer. J Am Soc Mass Spectrom 20:1451–1463
Powell DW, Pinchuk IV, Saada JI, Chen X, Mifflin RC (2011) Mesenchymal cells of the intestinal lamina propria. Annu Rev Physiol 73:213–237
Egeblad M, Nakasone ES, Werb Z (2010) Tumors as organs: complex tissues that interface with the entire organism. Dev Cell 18:884–901
Hakomori S (2000) Traveling for the glycosphingolipid path. Glycoconj J 17:627–647
Holst S, Stavenhagen K, Balog CIA, Koeleman CAM, McDonnell LM, Mayboroda OA, Verhoeven A, Mesker WE, Tollenaar RAEM, Deelder AM, Wuhrer M (2013) Investigations on aberrant glycosylation of glycosphingolipids in colorectal cancer tissues using liquid chromatography and matrix-assisted laser desorption time-of-flight mass spectrometry (MALDI-TOF-MS). Mol Cell Proteomics 12:3081–3093
Brulet M, Seyer A, Edelman A, Brunelle A, Fritsch J, Ollero M, Laprevote O (2010) Lipid mapping of colonic mucosa by cluster TOF-SIMS imaging and multivariate analysis in cftr knockout mice. J Lipid Res 51:3034–3045
Kurabe N, Hayasaka T, Ogawa M, Masaki N, Ide Y, Waki M, Nakamura T, Kurachi K, Kahyo T, Shinmura K, Midorikawa Y, Sugiyama Y, Setou M, Sugimura H (2013) Accumulated phosphatidylcholine (16:0/16:1) in human colorectal cancer; possible involvement of LPCAT4. Cancer Sci 104:1295–1302
Gerbig S, Golf O, Balog J, Denes J, Baranyai Z, Zarand A, Raso E, Timar J, Takats Z (2012) Analysis of colorectal adenocarcinoma tissue by desorption electrospray ionization mass spectrometric imaging. Anal Bioanal Chem 403:2315–2325
Acknowledgments
We are greatly thankful to the nurses and medical doctors of the Gastroenterology Unit of the Hospital Universitari de Son Espases (Palma, Spain) and of the Hospital Comarcal de Inca (Inca, Spain) for their participation during patient’s enrolment and sample acquisition. This study was partially supported by the Institute of Health Carlos III (Ministerio de Economía y Competitividad) and the EC (European Regional Development Fund, ERDF) (CP12/03338), by UPV/EHU (UFI 11/23), and by the Basque Government (SAIOTEK, consolidated groups). GBC and RRM’s contract were supported by the Miguel Servet program of the Institute of Health Carlos III. Technical and human support provided by the Servicio de Lipidómica of the SGIKER (UPV/EHU, MICINN, GV/EJ, ESF) is gratefully acknowledged.
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 329 kb)
Rights and permissions
About this article
Cite this article
Garate, J., Fernández, R., Lage, S. et al. Imaging mass spectrometry increased resolution using 2-mercaptobenzothiazole and 2,5-diaminonaphtalene matrices: application to lipid distribution in human colon. Anal Bioanal Chem 407, 4697–4708 (2015). https://doi.org/10.1007/s00216-015-8673-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-015-8673-7