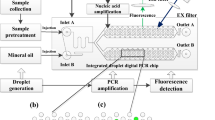
Abstract
Nucleic-acid amplification is a crucial step in nucleic-acid-sequence-detection assays. The use of digital microfluidic devices to miniaturize amplification techniques reduces the required sample volume and the analysis time and offers new possibilities for process automation and integration in a single device. The recently introduced droplet polymerase-chain-reaction (PCR) amplification methods require repeated cycles of two or three temperature-dependent steps during the amplification of the nucleic-acid target sequence. In contrast, low-temperature isothermal-amplification methods have no need for thermal cycling, thus requiring simplified microfluidic-device features. Here, the combined use of digital microfluidics and molecular-beacon (MB)-assisted isothermal circular-strand-displacement polymerization (ICSDP) to detect microRNA-210 sequences is described. MicroRNA-210 has been described as the most consistently and predominantly upregulated hypoxia-inducible factor. The nmol L−1–pmol L−1 detection capabilities of the method were first tested by targeting single-stranded DNA sequences from the genetically modified Roundup Ready soybean. The ability of the droplet-ICSDP method to discriminate between full-matched, single-mismatched, and unrelated sequences was also investigated. The detection of a range of nmol L−1–pmol L−1 microRNA-210 solutions compartmentalized in nanoliter-sized droplets was performed, establishing the ability of the method to detect as little as 10−18 mol of microRNA target sequences compartmentalized in 20 nL droplets. The suitability of the method for biological samples was tested by detecting microRNA-210 from transfected K562 cells.







Similar content being viewed by others
References
Schneider T, Kreutz J, Chiu DT (2013) The potential impact of droplet microfluidics in Biology. Anal Chem 85:3476–3482
Zanoli LM, Spoto G (2013) Isothermal amplification methods for the detection of nucleic Acids in microfluidic devices. Biosensors 3:18–43
Spoto G, Corradini R (2012) Detection of non-amplified genomic DNA. Springer, Verlag
Pinto AJ, Raskin L (2012) PCR biases distort bacterial and archaeal community structure in pyrosequencing datasets. Plos One 7:e43093
Craw P, Balachandran W (2012) Isothermal nucleic acid amplification technologies for point-of-care diagnostics: a critical review. Lab Chip 12:2469–2486
Asiello PJ, Baeumner AJ (2011) Miniaturized isothermal nucleic acid amplification, a review. Lab Chip 11:1420–1430
Kim J, Easley CJ (2011) Isothermal DNA amplification in bioanalysis: strategies and applications. Bioanalysis 3:227–239
Guo Q, Yang X, Wang K, Tan W, Li W, Tang H, Li H (2009) Sensitive fluorescence detection of nucleic acids based on isothermal circular strand-displacement polymerization reaction. Nucleic Acids Res 37(3):e20
Duan R, Zuo X, Wang S, Quan X, Chen D, Chen Z, Jiang L, Fan C, Xia F (2013) Lab in a Tube: Ultrasensitive detection of microRNAs at the single-cell level and in breast cancer patients using quadratic isothermal amplification. J Am Chem Soc 135(12):4604–4607
Duan R, Zuo X, Wang S, Quan X, Chen D, Chen Z, Jiang L, Fan C, Xia F (2014) Quadratic isothermal amplification for the detection of microRNA. Nat Protoc 9:597–607
Nana-Sinkam SP, Croce CM (2013) Clinical applications for microRNAs in cancer. Clin Pharmacol Ther 93:98–104
Mendell JT, Olson EN (2012) MicroRNAs in stress signaling and human disease. Cell 148:1172–1187
Zampetaki A, Mayr M (2012) MicroRNAs in vascular and metabolic disease. Circ Res 110:508–522
Bartel DP (2009) MicroRNAs: target recognition and regulatory functions. Cell 136:215–233
Dong H, Lei J, Ding L, Wen Y, Ju H, Zhang X (2013) MicroRNA: function, detection, and bioanalysis. Chem Rev 113:6207–6233
Shivdasani RA (2006) MicroRNAs: regulators of gene expression and cell differentiation. Blood 108:3646–3653
Tzimagiorgis G, Michailidou EZ, Kritis A, Markopoulos AK, Kouidou S (2011) Recovering circulating extracellular or cell-free RNA from bodily fluids. Cancer Epidemiol 35:580–589
Pritchard CC, Cheng HH, Tewari M (2012) MicroRNA profiling: approaches and considerations. Nat Rev Genet 13:358–369
D'Agata R, Breveglieri G, Zanoli LM, Borgatti M, Spoto G, Gambari R (2011) Direct detection of point mutations in non-amplified human genomic DNA. Anal Chem 83:8711–8717
D’Agata R, Spoto G (2013) Surface plasmon resonance imaging for nucleic acid detection. Anal Bioanal Chem 405:573–584
Zanoli LM, D’Agata R, Spoto G (2012) Functionalized gold nanoparticles for the ultrasensitive DNA detection. Anal Bioanal Chem 402:1759–1771
Spoto G, Minunni M (2012) Surface Plasmon Resonance Imaging: What Next? J Phys Chem Lett 3:2682–2691
Baker M (2012) Digital PCR hits its stride. Nat Methods 9:541–544
Hindson CM, Chevillet JR, Briggs HA, Gallichotte EN, Ruf IK, Hindson BJ, Vessella RL, Tewari M (2013) Absolute quantification by droplet digital PCR versus analog real-time PCR. Nat Methods 10:1003–1005
Witwer KW, McAlexander MA, Queen SE, Adams RJ (2013) Real-time quantitative PCR and droplet digital PCR for plant miRNAs in mammalian blood provide little evidence for general uptake of dietary miRNAs. RNA Biol 10:1080–1086
Shen F, Davydova EK, Du W, Kreutz JE, Piepenburg O, Ismagilov RF (2011) Digital isothermal quantification of nucleic acids via simultaneous chemical initiation of recombinase polymerase amplification reactions on SlipChip. Anal Chem 83:3533–3540
Gansen A, Herrick AM, Dimov IK, Leeb LP, Chiua DT (2012) Digital LAMP in a sample self-digitization (SD) chip. Lab Chip 12:2247–2254
Blainey PC, Quake SR (2011) Digital MDA for enumeration of total nucleic acid contamination. Nucleic Acids Res 39:e19
Konry T, Smolina I, Yarmush JM, Irimia D, Yarmush ML (2011) Ultrasensitive detection of low-abundance surface-marker protein using isothermal rolling circle amplification in a microfluidic nanoliter platform. Small 7:395–400
Mazutis L, Araghi AF, Miller OJ, Baret JC, Frenz L, Janoshazi A, Taly V, Miller BJ, Hutchison JB, Link D, Griffiths AD, Ryckelynck M (2009) Droplet-based microfluidic systems for high-throughput single DNA molecule isothermal amplification and analysis. Anal Chem 81:4813–4821
Fasanaro P, D'Alessandra Y, Di Stefano V, Melchionna R, Romani S, Pompilio G, Capogrossi MC, Martelli F (2008) MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand ephrin-A3. J Biol Chem 283:15878–15883
Manicardi A, Fabbri E, Tedeschi T, Sforza S, Bianchi N, Brognara E, Gambari R, Marchelli R, Corradini R (2012) Cellular uptakes, biostabilities and anti-miR-210 activities of chiral arginine-PNAs in leukaemic K562 cells. Chem Biochem 13:1327–1337
Fabbri E, Manicardi A, Tedeschi T, Sforza S, Bianchi N, Brognara E, Finotti A, Breveglieri G, Borgatti M, Corradini R, Marchelli R, Gambari R (2011) Modulation of the biological activity of microRNA-210 with peptide nucleic acids (PNAs). Chem Med Chem 6:2192–2202
Whitehead CL, Teh WT, Walker SP, Leung C, Larmour L, Tong S (2013) Circulating MicroRNAs in maternal blood as potential biomarkers for fetal hypoxia in-utero. PLoS One 8:e78487
Røsjø H, Dahl MB, Bye A, Andreassen J, Jørgensen M, Wisløff U, Christensen G, Edvardsen T, Omland T (2014) Prognostic value of circulating microRNA-210 levels in patients with moderate to severe aortic stenosis. PLoS One 9:e91812
Zhao A, Li G, Péoc'h M, Genin C, Gigante M (2013) Serum miR-210 as a novel biomarker for molecular diagnosis of clear cell renal cell carcinoma. Exp Mol Pathol 94:115–120
Ho AS, Huang X, Cao H, Christman-Skieller C, Bennewith K, Le QT, Koong AC (2010) Circulating miR-210 as a Novel Hypoxia Marker in Pancreatic Cancer. Transl Oncol 3:109–113
Cheng HH, Mitchell PS, Kroh EM, Dowell AE, Chéry L, Siddiqui J, Nelson PS, Vessella RL, Knudsen BS, Chinnaiyan AM, Pienta KJ, Morrissey C, Tewari M (2013) Circulating microRNA profiling identifies a subset of metastatic prostate cancer patients with evidence of cancer-associated hypoxia. PLoS One 8:e69239
Jung EJ, Santarpia L, Kim J, Esteva FJ, Moretti E, Buzdar AU, Di Leo A, Le XF, Bast RC Jr, Park ST, Pusztai L, Calin GA (2012) Plasma microRNA 210 levels correlate with sensitivity to trastuzumab and tumor presence in breast cancer patients. Cancer 118:2603–2614
D’Agata R, Corradini R, Ferretti C, Zanoli L, Gatti M, Marchelli R, Spoto G (2010) Ultrasensitive detection of non-amplified genomic DNA by nanoparticle-enhanced surface plasmon resonance imaging. Biosens Bioelectron 25:2095–2100
Grasso G, D'Agata R, Zanoli L, Spoto G (2009) Microfluidic networks for surface plasmon resonance imaging real-time kinetics experiments. Microchem J 93:82–86
Tice JD, Lyon AD, Ismagilov RF (2004) Effects of viscosity on droplet formation and mixing inmicrofluidic channels. Anal Chim Acta 507:73–77
Yin BC, Liu YQ, Ye BC (2013) Sensitive detection of microRNA in complex biological samples via enzymatic signal amplification using DNA polymerase coupled with nicking endonuclease. Anal Chem 85:11487–11493
Bi S, Zhang J, Hao S, Ding C, Zhang S (2011) Exponential amplification for chemiluminescence resonance energy transfer detection of microRNA in real samples based on a cross-catalyst strand-displacement network. Anal Chem 83:3696–3702
Persat A, Chivukula RR, Mendell JT, Santiago JG (2010) Quantification of global microRNA abundance by selective isotachophoresis. Anal Chem 82:9631–9635
Wang J, Yi X, Tang H, Han H, Wu M, Zhou F (2012) Direct quantification of microRNA at low picomolar level in sera of glioma patients using a competitive hybridization followed by amplified voltammetric detection. Anal Chem 84:6400–6406
Acknowledgments
MIUR (PRIN 20093N774P), Ministry of Health, Italy (n. 098/GR-2009-1596647), COST Action TD1003-Bio-inspired nanotechnologies: from concepts to applications and the Italian Association for Cancer Research (AIRC 13575: Peptide nucleic acids targeting oncomiR and tumor-suppressor miRNAs: cancer diagnosis and therapy) are acknowledged for partial financial support.
Author information
Authors and Affiliations
Corresponding author
Additional information
ABC Highlights: authored by Rising Stars and Top Experts
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 310 kb)
Rights and permissions
About this article
Cite this article
Giuffrida, M.C., Zanoli, L.M., D’Agata, R. et al. Isothermal circular-strand-displacement polymerization of DNA and microRNA in digital microfluidic devices. Anal Bioanal Chem 407, 1533–1543 (2015). https://doi.org/10.1007/s00216-014-8405-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-8405-4