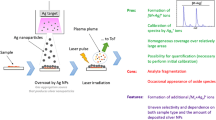
Abstract
The surface chemistry of gold nanowires (AuNWs) has been systematically assessed in terms of contamination and cleaning processes. The nanomaterial’s surface quality was correlated to its performance in the matrix-free laser desorption ionization mass spectrometry (LDI-MS) analysis of low molecular weight analytes. Arrays of AuNWs were deposited on glass slides by means of the lithographically patterned nanowire electrodeposition technique. AuNWs were then characterized in terms of surface chemical composition and morphology using X-ray photoelectron spectroscopy, scanning electron microscopy and atomic force microscopy. AuNWs were subjected to a series of well-known cleaning procedures with the aim of producing the best performing surfaces for the LDI-MS detection of leucine enkephalin, chosen as a model analyte with a molar mass below 1,000 g/mol. Prolonged cyclic voltammetry in 2 M sulfuric acid and, most of all, oxygen plasma cleaning for 5 min provided the best results in terms of simpler (interference-free) and more intense mass spectrometry spectra of the reference compound. The analyte always ionized as the sodiated adduct, and leucine enkephalin limits of detection of 0.5 and 2.5 pmol were estimated for the positive and negative analysis modes, respectively. This study points out the tight correlation existing between the chemical status of the nanostructure surface and the AuNW-assisted LDI-MS performance in terms of reproducibility of spectra, intensity of analyte ions and reduction of interferences.

SEM (a-d) and AFM (e-f) pictures and LDI-MS spectra of leu-enk analyte (g-h) obtained with untreated (left side) and oxygen plasmatreated (right side) gold nanowire arrays supported on glass slide






Similar content being viewed by others
References
McLean JA, Stumpo KA, Russell DH (2005) Size-selected (2–10 nm) gold nanoparticles for matrix assisted laser desorption ionization of peptides. J Am Chem Soc 127:5304–5305. doi:10.1021/ja043907w
Pilolli R, Palmisano F, Cioffi N (2012) Gold nanomaterials as a new tool for bioanalytical applications of laser desorption ionization mass spectrometry. Anal Bioanal Chem 402:601–623. doi:10.1007/s00216-011-5120-2
Chiang C-K, Chen W-T, Chang H-T (2011) Nanoparticle-based mass spectrometry for the analysis of biomolecules. Chem Soc Rev 40:1269–1281. doi:10.1039/C0CS00050G
Qiao L, Liu B, Girault HH (2010) Nanomaterial-assisted laser desorption ionization for mass spectrometry-based biomedical analysis. Nanomedicine 5:1641–1652. doi:10.2217/nnm.10.127
Arakawa R, Kawasaki H (2010) Functionalized nanoparticles and nanostructured surfaces for surface-assisted laser desorption/ionization mass spectrometry. Anal Sci 26:1229–1240
Law KP, Larkin J (2011) Recent advances in SALDI-MS techniques and their chemical and bioanalytical applications. Anal Bioanal Chem 399:2597–2622. doi:10.1007/s00216-010-4063-3
Najam-ul-Haq M, Jabeen F, Hussain D, Saeed A, Musharraf SG, Huck CW, Bonn GK (2012) Versatile nanocomposites in phosphoproteomics: a review. Anal Chim Acta 747:7–18. doi:10.1016/j.aca.2012.08.004
Silina YE, Volmer DA (2013) Nanostructured solid substrates for efficient laser desorption/ionization mass spectrometry (LDI-MS) of low molecular weight compounds. Analyst 138:7053–7065. doi:10.1039/C3AN01120H
Pingarrón JM, Yáñez-Sedeño P, González-Cortés A (2008) Gold nanoparticle-based electrochemical biosensors. Electrochim Acta 53:5848–5866. doi:10.1016/j.electacta.2008.03.005
Huang X, Jain PK, El-Sayed IH, El-Sayed MA (2007) Gold nanoparticles: interesting optical properties and recent applications in cancer diagnostics and therapy. Nanomedicine 2:681–693. doi:10.2217/17435889.2.5.681
Jiang X-M, Wang L-M, Wang J, Chen C-Y (2012) Gold nanomaterials: preparation, chemical modification, biomedical applications and potential risk assessment. Appl Biochem Biotechnol 166:1533–1551. doi:10.1007/s12010-012-9548-4
Colaianni L, Kung SC, Taggart DK, De Giorgio V, Greaves J, Penner RM, Cioffi N (2010) Laser desorption ionization-mass spectrometry detection of amino acids and peptides promoted by gold nanowires. Sens Lett 8:539–544. doi:10.1166/sl.2010.1308
Amendola V, Litti L, Meneghetti M (2013) LDI-MS assisted by chemical-free gold nanoparticles: enhanced sensitivity and reduced background in the low-mass region. Anal Chem 85:11747–11754. doi:10.1021/ac401662r
Pilolli R, Ditaranto N, Di Franco C, Palmisano F, Cioffi N (2012) Thermally annealed gold nanoparticles for surface-assisted laser desorption ionisation–mass spectrometry of low molecular weight analytes. Anal Bioanal Chem 404:1703–1711. doi:10.1007/s00216-012-6243-9
Pilolli R, Ditaranto N, Monopoli A, Nacci A, Palmisano F, Sabbatini L, Cioffi N (2014) Designing functionalized gold surfaces and nanostructures for laser desorption ionisation mass spectrometry. Vacuum 100:78–83. doi:10.1016/j.vacuum.2013.07.032
Jin J, Choi S, Kim Y, Choi M, Kim J, Kim S (2012) Evaluation of nanoporous gold with controlled surface structures for laser desorption ionization (LDI) analysis: surface area versus LDI signal intensity. J Am Soc Mass Spectrom 23:1450–1453. doi:10.1007/s13361-012-0439-2
Chiu W-C, Huang C-C (2013) Combining fibrinogen-conjugated gold nanoparticles with a cellulose membrane for the mass spectrometry-based detection of fibrinolytic-related proteins. Anal Chem 85:6922–6929. doi:10.1021/ac4013418
Liu Y-C, Chang H-T, Chiang C-K, Huang C-C (2012) Pulsed-laser desorption/ionization of clusters from biofunctional gold nanoparticles: implications for protein detections. ACS Appl Mater Interfaces 4:5241–5248. doi:10.1021/am3011934
Seo H, Kim S, Kim JI, Kang H, Jung W, Yeo W-S (2013) Ultrasensitive detection of microRNAs using nanoengineered micro gold shells and laser desorption/ionization time-of-flight MS. Anal Biochem 434:199–201. doi:10.1016/j.ab.2012.11.009
Liu Y-C, Chiang C-K, Chang H-T, Lee Y-F, Huang C-C (2011) Using a functional nanogold membrane coupled with laser desorption/ionization mass spectrometry to detect lead ions in biofluids. Adv Funct Mater 21:4448–4455. doi:10.1002/adfm.201101248
Kim Y-K, Min D-H (2012) Preparation of the hybrid film of poly(allylamine hydrochloride)-functionalized graphene oxide and gold nanoparticle and its application for laser-induced desorption/ionization of small molecules. Langmuir 28:4453–4458. doi:10.1021/la204185p
Creran B, Yan B, Moyano DF, Gilbert MM, Vachet RW, Rotello VM (2012) Laser desorption ionization mass spectrometric imaging of mass barcoded gold nanoparticles for security applications. Chem Commun 48:4543–4545. doi:10.1039/C2CC30499F
Tseng Y-T, Chang H-Y, Huang C-C (2012) A mass spectrometry-based immunosensor for bacteria using antibody-conjugated gold nanoparticles. Chem Commun 48:8712–8714. doi:10.1039/C2CC34120D
Castellana ET, Russell DH (2007) Tailoring nanoparticle surface chemistry to enhance laser desorption ionization of peptides and proteins. Nano Lett 7:3023–3025. doi:10.1021/nl071469w
Nayak R, Knapp DR (2010) Matrix-Free LDI Mass Spectrometry Platform Using Patterned Nanostructured Gold Thin Film. Anal Chem 82:7772–7778. doi:10.1021/ac1017277
Nakamura Y, Tsuru Y, Fujii M, Taga Y, Kiya A, Nakashima N, Niidome Y (2011) Sensing of oligopeptides using localized surface plasmon resonances combined with surface-assisted laser desorption/ionization time-of-flight mass spectrometry. Nanoscale 3:3793–3798. doi:10.1039/C1NR10519A
Hsieh Y-T, Chen W-T, Tomalová I, Preisler J, Chang H-T (2012) Detection of melamine in infant formula and grain powder by surface-assisted laser desorption/ionization mass spectrometry. Rapid Commun Mass Spectrom 26:1393–1398. doi:10.1002/rcm.6238
Liu M, Zhang L, Xu Y, Yang P, Lu H (2013) Mass spectrometry signal amplification for ultrasensitive glycoprotein detection using gold nanoparticle as mass tag combined with boronic acid based isolation strategy. Anal Chim Acta 788:129–134. doi:10.1016/j.aca.2013.05.063
Wang L, Wei G, Sun L, Liu Z, Song Y, Yang T, Sun Y, Guo C, Li Z (2006) Self-assembly of cinnamic acid-capped gold nanoparticles. Nanotechnology 17:2907
Yonezawa T, Kawasaki H, Tarui A, Watanabe T, Arakawa R, Shimada T, Mafune F (2009) Detailed investigation on the possibility of nanoparticles of various metal elements for surface-assisted laser desorption/ionization mass spectrometry. Anal Sci 25:339–346
Menke EJ, Thompson MA, Xiang C, Yang LC, Penner RM (2006) Lithographically patterned nanowire electrodeposition. Nat Mater 5:914–919. doi:10.1038/nmat1759
Xiang C, Kung S-C, Taggart DK, Yang F, Thompson MA, Güell AG, Yang Y, Penner RM (2008) Lithographically patterned nanowire electrodeposition: a method for patterning electrically continuous metal nanowires on dielectrics. ACS Nano 2:1939–1949. doi:10.1021/nn800394k
Morita C, Tanuma H, Kawai C, Ito Y, Imura Y, Kawai T (2013) Room-temperature synthesis of two-dimensional ultrathin gold nanowire parallel array with tunable spacing. Langmuir 29:1669–1675. doi:10.1021/la304925e
Hsieh Y-T, Sun I-W (2014) One-step electrochemical fabrication of nanoporous gold wire arrays from ionic liquid. Chem Commun 50:246–248. doi:10.1039/C3CC46061D
Chen Y-L, Lee C-Y, Chiu H-T (2013) Growth of gold nanowires on flexible substrate for highly sensitive biosensing: detection of thrombin as an example. J Mater Chem B 1:186–193. doi:10.1039/C2TB00010E
Zhang M, Yang X, Zhou Z, Ye X (2013) Controllable growth of gold nanowires and nanoactuators via high-frequency AC electrodeposition. Electrochem Commun 27:133–136. doi:10.1016/j.elecom.2012.11.013
Kundu S (2013) A new route for the formation of Au nanowires and application of shape-selective Au nanoparticles in SERS studies. J Mater Chem C 1:831–842. doi:10.1039/C2TC00315E
Yang L, Zhang Y, Chu M, Deng W, Tan Y, Ma M, Su X, Xie Q, Yao S (2014) Facile fabrication of network film electrodes with ultrathin Au nanowires for nonenzymatic glucose sensing and glucose/O2 fuel cell. Biosens Bioelectron 52:105–110. doi:10.1016/j.bios.2013.08.038
Gedamu D, Jebril S, Schuchardt A, Elbahri M, Wille S, Mishra YK, Adelung R (2010) Examples for the integration of self-organized nanowires for functional devices by a fracture approach. Phys Status Solidi B 247(10):2571–2580
Mishra YK, Kabiraj D, Sulania I, Pivin JC, Avasthi DK (2007) Synthesis and characterization of gold nanorings. J Nanosci Nanotechnol 7:1878–1881
Liu R, Liu J, Zhou X, Jiang G (2011) Cysteine modified small ligament Au nanoporous film: an easy fabricating and highly efficient surface-assisted laser desorption/ionization substrate. Anal Chem 83:3668–3674. doi:10.1021/ac103222p
Sztáray J, Memboeuf A, Drahos L, Vékey K (2011) Leucine enkephalin—a mass spectrometry standard. Mass Spectrom Rev 30:298–320. doi:10.1002/mas.20279
Patti GJ, Woo HK, Yanes O, Shriver L, Thomas D, Uritboonthai W, Apon JV, Steenwyk R, Manchester M, Siuzdak G (2010) Detection of carbohydrates and steroids by cation-enhanced nanostructure-initiator mass spectrometry (NIMS) for biofluid analysis and tissue imaging. Anal Chem 82:121–128
Wu HP, Yu CJ, Lin CY, Lin YH, Tseng WL (2009) Gold nanoparticles as assisted matrices for the detection of biomolecules in a high-salt solution through laser desorption/ionization mass spectrometry. J Am Soc Mass Spectrom 20:875–882
Tsuji T, Mizuki T, Yasumoto M, Tsuji M, Kawasaki H, Yonezawa T, Mafuné F (2011) Efficient fabrication of substrates for surface-assisted laser desorption/ionization mass spectrometry using laser ablation in liquids. Appl Surf Sci 6:2046–2050. doi:10.1016/j.apsusc.2010.08.128
Vertes A (2012) Laser–nanostructure interactions for ion production. Phys Chem Chem Phys 14:8453–8471
Spencer MT, Furutani H, Oldenburg SJ, Darlington TK, Prather KA (2008) Gold nanoparticles as a matrix for visible-wavelength single-particle matrix-assisted laser desorption/ionization mass spectrometry of small biomolecules. J Phys Chem C 112:4083–4090
Luo G, Marginean I, Vertes A (2002) Internal energy of ions generated by matrix-assisted laser desorption/ionization. Anal Chem 74:6185–6190
Greisch J-F, Gabelica V, Remacle F, De Pauw E (2003) Thermometer ions for matrix-enhanced laser desorption/ionization internal energy calibration. Rapid Commun Mass Spectrom 17:1847–1854
Acknowledgments
N.C., L.C. and R.A.P. acknowledge the financial support from the Italian Project “Nanomaterials & Laser Ionization Mass Spectrometry: A New Bio-analytical Approach” FIRB Futuro in Ricerca 2008, funded by the Ministero dell’Istruzione, dell’Università e della Ricerca. S.C.K., D.K.T and R.M.P. acknowledge the financial support of this work through the US National Science Foundation (contract CHE 1306928). N.C. warmly thanks F. Palmisano for scientific discussions on MS experiments.
Author information
Authors and Affiliations
Corresponding author
Additional information
ABC Highlights: authored by Rising Stars and Top Experts
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 4.74 mb)
Rights and permissions
About this article
Cite this article
Colaianni, L., Kung, S.C., Taggart, D.K. et al. Reduction of spectral interferences using ultraclean gold nanowire arrays in the LDI-MS analysis of a model peptide. Anal Bioanal Chem 406, 4571–4583 (2014). https://doi.org/10.1007/s00216-014-7876-7
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-014-7876-7