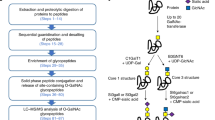
Abstract
Hog or porcine gastric mucin resembles the human source in carrying not only blood group antigens but also the rather rare α4-GlcNAc-capped terminal epitope functionally implicated in protection against Helicobacter pylori infection. Being more readily available and reasonably well characterized, it serves as a good reagent for immunobiological studies, as well as a standard for analytical methodology developments. Current approaches in mass spectrometry (MS)-based glycomic mapping remain vastly inadequate in revealing the full complexity of glycosylation, particularly for cases such as the extremely heterogeneous O-glycosylation of mucosal mucins that can be further sulfated. We demonstrate here a novel concerted workflow that extends the conventional matrix-assisted laser desorption/ionization–mass spectrometry (MALDI-MS) mapping of permethylated glycans in positive ion mode to include a further step of sulfoglycomic analysis in negative ion mode. This was facilitated by introducing a mixed-mode solid-phase extraction step, which allows direct cleanup and simultaneous fractionation of the permethylated glycans into separate non-sulfated and sulfated pools in one single step. By distinct MALDI-MS/MS fragmentation patterns, all previously known structural features of porcine gastric mucin including the terminal epitopes and location of sulfates could be readily defined. We additionally showed that both arms of the core 2 structures could be extended via 6-O-sulfated GlcNAc to yield a series of disulfated O-glycans not previously reported, thus expanding its current glycomic coverage. However, a targeted LC-MSn analysis was required and best suited to dig even deeper into validating the occurrence of very minor structural isomers carrying the Lewis Y epitope implicated by positive antibody binding.






Similar content being viewed by others
Abbreviations
- CID:
-
Collision-induced dissociation
- lacdiNAc:
-
N,N′-Diacetyllactosamine
- lacNAc:
-
N-Acetyllactosamine
- mAb:
-
Monoclonal antibody
- MS:
-
Mass spectrometry
- SPE:
-
Solid-phase extraction
- TFA:
-
Trifluoroacetic acid
References
Moremen KW, Tiemeyer M, Nairn AV (2012) Vertebrate protein glycosylation: diversity, synthesis and function. Nat Rev Mol Cell Biol 13:448–462
Zaia J (2010) Mass spectrometry and glycomics. OMICS 14:401–418
North SJ, Hitchen PG, Haslam SM, Dell A (2009) Mass spectrometry in the analysis of N-linked and O-linked glycans. Curr Opin Struct Biol 19:498–506
Hayes CA, Nemes S, Issa S, Jin C, Karlsson NG (2012) Glycomic work-flow for analysis of mucin O-linked oligosaccharides. Methods Mol Biol 842:141–163
McGuckin MA, Linden SK, Sutton P, Florin TH (2011) Mucin dynamics and enteric pathogens. Nat Rev Microbiol 9:265–278
Johansson ME, Ambort D, Pelaseyed T, Schutte A, Gustafsson JK, Ermund A, Subramani DB, Holmen-Larsson JM, Thomsson KA, Bergstrom JH, van der Post S, Rodriguez-Pineiro AM, Sjovall H, Backstrom M, Hansson GC (2011) Composition and functional role of the mucus layers in the intestine. Cell Mol Life Sci 68:3635–3641
Johansson ME, Sjovall H, Hansson GC (2013) The gastrointestinal mucus system in health and disease. Nat Rev Gastroenterol Hepatol 10: 352–361
Slomiany BL, Meyer K (1973) Oligosaccharides produced by acetolysis of blood group active(A + H)sulfated glycoproteins from hog gastric mucin. J Biol Chem 248:2290–2295
Kochetkov NK, Derevitskaya VA, Arbatsky NP (1976) The structure of pentasaccharides and hexasaccharides from blood group substance H. Eur J Biochem 67:129–136
Derevitskaya VA, Arbatsky NP, Kochetkov NK (1978) The structure of carbohydrate chains of blood-group substance. Isolation and elucidation of the structure of higher oligosaccharides from blood-group substance H, Eur J Biochem 86:423–437
Van Halbeek H, Dorland L, Vliegenthart JF, Kochetkov NK, Arbatsky NP, Derevitskaya VA (1982) Characterization of the primary structure and the microheterogeneity of the carbohydrate chains of porcine blood-group H substance by 500-MHz 1H-NMR spectroscopy. Eur J Biochem 127:21–29
Zenteno E, Vazquez L, Chavez R, Cordoba F, Wieruszeski JM, Montreuil J, Debray H (1995) Specificity of the isolectins from the plant cactus Machaerocereus eruca for oligosaccharides from porcine stomach mucin. Glycoconj J 12:699–706
Karlsson NG, Nordman H, Karlsson H, Carlstedt I, Hansson GC (1997) Glycosylation differences between pig gastric mucin populations: a comparative study of the neutral oligosaccharides using mass spectrometry. Biochem J 326(Pt 3):911–917
Thomsson KA, Karlsson NG, Hansson GC (1999) Liquid chromatography-electrospray mass spectrometry as a tool for the analysis of sulfated oligosaccharides from mucin glycoproteins. J Chromatogr A 854:131–139
Thomsson KA, Karlsson H, Hansson GC (2000) Sequencing of sulfated oligosaccharides from mucins by liquid chromatography and electrospray ionization tandem mass spectrometry. Anal Chem 72:4543–4549
Rossez Y, Maes E, Lefebvre Darroman T, Gosset P, Ecobichon C, Joncquel Chevalier Curt M, Boneca IG, Michalski JC, Robbe-Masselot C (2012) Almost all human gastric mucin O-glycans harbor blood group A, B or H antigens and are potential binding sites for Helicobacter pylori. Glycobiology 22:1193–1206
Nakayama J, Yeh JC, Misra AK, Ito S, Katsuyama T, Fukuda M (1999) Expression cloning of a human alpha1, 4-N-acetylglucosaminyltransferase that forms GlcNAcalpha1-->4Galbeta-->R, a glycan specifically expressed in the gastric gland mucous cell-type mucin. Proc Natl Acad Sci U S A 96:8991–8996
Ishihara K, Kurihara M, Goso Y, Urata T, Ota H, Katsuyama T, Hotta K (1996) Peripheral alpha-linked N-acetylglucosamine on the carbohydrate moiety of mucin derived from mammalian gastric gland mucous cells: epitope recognized by a newly characterized monoclonal antibody. Biochem J 318(Pt 2):409–416
Kawakubo M, Ito Y, Okimura Y, Kobayashi M, Sakura K, Kasama S, Fukuda MN, Fukuda M, Katsuyama T, Nakayama J (2004) Natural antibiotic function of a human gastric mucin against Helicobacter pylori infection. Science 305:1003–1006
Lee H, Wang P, Hoshino H, Ito Y, Kobayashi M, Nakayama J, Seeberger PH, Fukuda M (2008) Alpha1,4GlcNAc-capped mucin-type O-glycan inhibits cholesterol alpha-glucosyltransferase from Helicobacter pylori and suppresses H. pylori growth. Glycobiology 18:549–558
Karasawa F, Shiota A, Goso Y, Kobayashi M, Sato Y, Masumoto J, Fujiwara M, Yokosawa S, Muraki T, Miyagawa S, Ueda M, Fukuda MN, Fukuda M, Ishihara K, Nakayama J (2012) Essential role of gastric gland mucin in preventing gastric cancer in mice. J Clin Invest 122:923–934
Kenny DT, Skoog EC, Linden SK, Struwe WB, Rudd PM, Karlsson NG (2012) Presence of terminal N-acetylgalactosaminebeta1-4N-acetylglucosamine residues on O-linked oligosaccharides from gastric MUC5AC: Involvement in Helicobacter pylori colonization? Glycobiology 22:1077–1085
Thomsson KA, Backstrom M, Holmen Larsson JM, Hansson GC, Karlsson H (2010) Enhanced detection of sialylated and sulfated glycans with negative ion mode nanoliquid chromatography/mass spectrometry at high pH. Anal Chem 82:1470–1477
Khoo KH, Yu SY (2010) Mass spectrometric analysis of sulfated N- and O-glycans. Methods Enzymol 478:3–26
Wu AM, Lin SR, Chin LK, Chow LP, Lin JY (1992) Defining the carbohydrate specificities of Abrus precatorius agglutinin as T (Gal beta 1--3GalNAc) greater than I/II (Gal beta 1--3/4GlcNAc). J Biol Chem 267:19130–19139
Wu AM, Khoo KH, Yu SY, Yang Z, Kannagi R, Watkins WM (2007) Glycomic mapping of pseudomucinous human ovarian cyst glycoproteins: Identification of Lewis and sialyl Lewis glycotopes. Proteomics. Proteomics 7:3699–3717
Yu SY, Wu SW, Khoo KH (2006) Distinctive characteristics of MALDI-Q/TOF and TOF/TOF tandem mass spectrometry for sequencing of permethylated complex type N-glycans. Glycoconj J 23:355–369
Yu SY, Wu SW, Hsiao HH, Khoo KH (2009) Enabling techniques and strategic workflow for sulfoglycomics based on mass spectrometry mapping and sequencing of permethylated sulfated glycans. Glycobiology 19:1136–1149
Patnode ML, Yu SY, Cheng CW, Ho MY, Tegesjo L, Sakuma K, Uchimura K, Khoo KH, Kannagi R, Rosen SD (2013) KSGal6ST generates galactose-6-O-sulfate in high endothelial venules but does not contribute to L-selectin-dependent lymphocyte homing. Glycobiology 23:381–394
Duk M, Lisowska E, Wu JH, Wu AM (1994) The biotin/avidin-mediated microtiter plate lectin assay with the use of chemically modified glycoprotein ligand. Anal Biochem 221:266–272
Lisowska E, Duk M, Wu AM (1996) Preparation of biotinylated lectins and application in microtiter plate assays adn Western blotting. Bio Methods. Bio Methods 7:115–129
Yu SY, Yang Z, Khoo KH, Wu AM (2009) Identification of blood group A/A-Leb/y and B/B-Leb/y active glycotopes co-expressed on the O-glycans isolated from two distinct human ovarian cyst fluids. Proteomics 9:3445–3462
Acknowledgments
This work was supported by Academia Sinica and Taiwan National Science Council (NSC) grant 99-2311-B-001-021-MY3 (to KKH), NSC grant 101-2320-B-182-011, and Chang-Gung Medical Research Project (CMRP) grant nos. 180482 and 170443 (to WAM). The MS data were acquired at the previous NRPGM Core Facilities for Proteomics and Glycomics (NSC 99-3112-B-001-025), and current Core Facilities for Protein Structural Analysis at Academia Sinica, supported under the Taiwan National Core Facility Program for Biotechnology, NSC 100-2325-B-001-029, NSC101-2319-B-001-003. The authors thank Ms. Yu-Ping Gong for technical assistance and data collection.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Cheng, PF., Snovida, S., Ho, MY. et al. Increasing the depth of mass spectrometry-based glycomic coverage by additional dimensions of sulfoglycomics and target analysis of permethylated glycans. Anal Bioanal Chem 405, 6683–6695 (2013). https://doi.org/10.1007/s00216-013-7128-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-013-7128-2