Abstract
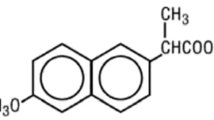
A sensitive LC-MS/MS assay for quantification of total and free concentrations of R- and S-warfarin in plasma was required to support clinical studies on warfarin enantiomers. Several ultrafiltration devices were evaluated for separation of free warfarin from plasma proteins. The highest precision and lowest non-specific binding was obtained for Centrifree ultrafiltration devices. R- and S-warfarin were extracted from plasma (total) and ultrafiltrate (free) by liquid–liquid extraction with methyl tert-butyl ether using d6-warfarin as internal standard. Mean extraction recovery was 68 ± 4%. The enantiomers were separated on a Chirobiotic V column with isocratic elution using 40% methanol and 0.03% acetic acid in water. Negative mode electrospray ionisation was used for MS/MS detection, monitoring the ion transition m/z 307/161. Calibration curves (quadratic, weighted 1/x) were fitted over the range of 20–2,000 ng/ml (r 2 ≥ 0.995) in plasma and 0.5–20 ng/ml (r 2 ≥ 0.998) in ultrafiltrate. The lower limit of quantification for R- and S-warfarin was 0.5 ng/ml in ultrafiltrate. Intra- and interday precision (% RSD) and bias were within 10% in all cases, and matrix effects were negligible. The assay was applied successfully to analysis of samples from clinical studies.

LC-MS/MS chromatogram of free R- and S-warfarin in human ultrafiltrate


Similar content being viewed by others
References
Dollery C (ed) (1999) Therapeutic drugs, 2nd edn. Churchill Livingstone, Edinburgh
Huang C, Yang J, Du Y, Miao L (2008) Measurement of free concentrations of highly protein-bound warfarin in plasma by ultra performance liquid chromatography-tandem mass spectrometry and its correlation with the international normalized ratio. Clin Chim Acta 393(2):85–89
Jones DR, Boysen G, Miller GP (2011) Novel multi-mode ultra performance liquid chromatography-tandem mass spectrometry assay for profiling enantiomeric hydroxywarfarins and warfarin in human plasma. J Chrom B 879(15–16):1056–1062
Zuo Z, Wo SK, Lo CMY, Zhou L, Cheng G, You JHS (2010) Simultaneous measurement of S-warfarin, R-warfarin, S-7-hydroxywarfarin and R-7-hydroxywarfarin in human plasma by liquid chromatography-tandem mass spectrometry. J Pharm Biomed Anal 52(2):305–310
Vecchione G, Casetta B, Tomaiuolo M, Grandone E, Margaglione M (2007) A rapid method for the quantification of the enantiomers of warfarin, phenprocoumon and acenocoumarol by two-dimensional-enantioselective liquid chromatography/electrospray tandem mass spectrometry. J Chrom B 850(1–2):507–514
Naidong W, Ring PR, Midtlien C, Jiang X (2001) Development and validation of a sensitive and robust LC-tandem MS method for the analysis of warfarin enantiomers in human plasma. J Pharm Biomed Anal 25(2):219–226
Radwan MA, Bawazeer GA, Aloudah NM, AlQuadeib BT, Aboul-Enein HY (2011) Determination of free and total warfarin concentrations in plasma using UPLC MS/MS and its application to a patient samples. Biomed Chrom. doi:10.1002/bmc.1616
Takahashi H, Kashima T, Kimura S, Muramoto N, Nakahata H, Kubo S, Shimoyama Y, Kajiwara M, Echizen H (1997) Determination of unbound warfarin enantiomers in human plasma and 7-hydroxywarfarin in human urine by chiral stationary-phase liquid chromatography with ultraviolet or fluorescence and on-line circular dichroism detection. J Chrom B 701(1):71–80
US Department of Health and Human Services (2001) Guidance for industry: bioanalytical method validation. US FDA Center for Drug Development and Research, Silver Spring
Uno T, Niioka T, Hayakari M, Sugawara K, Tateishi T (2007) Simultaneous determination of warfarin enantiomers and its metabolite in human plasma by column-switching high-performance liquid chromatography with chiral separation. Ther Drug Monit 29(3):333–339
Miura M, Okuyama S, Kato S, Kagaya H, Murata A, Komatsuda A, Wakui H, Sawada K (2011) Simultaneous determination of warfarin and 7-hydroxywarfarin enantiomers by high-performance liquid chromatography with ultraviolet detection. Ther Drug Monit 33(1):108–114
Yacobi A, Levy G (1977) Protein binding of warfarin enantiomers in serum of humans and rats. J Pharmacokinet Biopharm 5(2):123–131
Takahashi H, Kashima T, Nomizo Y, Muramoto N, Shimizu T, Nasu K, Kubota T, Kimura S, Echizen H (1998) Metabolism of warfarin enantiomers in Japanese patients with heart disease having different CYP2C9 and CYP2C19 genotypes. Clin Pharmacol Ther 63(5):519–528
Acknowledgements
The authors wish to acknowledge the New Zealand Lottery Grants Board and Health Research Council of New Zealand (ref. no. 08/322) for financial support of this study.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Jensen, B.P., Chin, P.K.L. & Begg, E.J. Quantification of total and free concentrations of R- and S-warfarin in human plasma by ultrafiltration and LC-MS/MS. Anal Bioanal Chem 401, 2187–2193 (2011). https://doi.org/10.1007/s00216-011-5303-x
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00216-011-5303-x