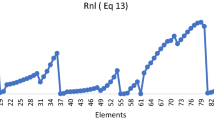
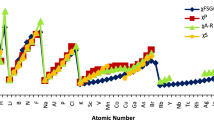
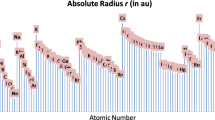
Abstract
Atomic hardness is an important periodic descriptor which can govern chemical reactivity and stability. A number of theoretical models are available to compute atomic hardness. In this report, we have suggested a new and simple approach to compute atomic hardness. Considering periodic relationship of atomic hardness with nucleophilicity index, effective nuclear charge and atomic radius, this model is derived to compute hardness of 103 elements of the periodic table. Our proposed scale satisfies all sine qua non of the periodic table. Characteristic periodic properties viz. lanthanide contraction, chemical inertness of noble gases, relativistic effect is quite distinct in our computed result. We have also calculated molecular hardness invoking Hardness Equalization Principle. A strong correlation between our computed data and their experimental counterparts justifies our study.




Similar content being viewed by others
References
Parr RG, Pearson RG (1983) J Am Chem Soc 105:7512. https://doi.org/10.1021/ja00364a005
Parr RG, Donnelly RA, Levy M, Palke W (1978) J Chem Phys 68:3801. https://doi.org/10.1063/1.436185
Parr RG, Yang W (1984) J Am Chem Soc 106:4049. https://doi.org/10.1021/ja00326a036
Islam N, Ghosh DC (2011) Mol Phys 109:1533. https://doi.org/10.1080/00268976.2011.569513
Pearson RG (1997) Chemical hardness: application from molecules to solid. Wiley-VCH, Weinheim
Mulliken RS (1952) J Am Chem Soc 74:811. https://doi.org/10.1021/ja01123a067
Pearson RG (1963) J Am Chem Soc 85:3533. https://doi.org/10.1021/ja00905a001
Pearson RG (1966) Science 151:172. https://www.jstor.org/stable/1717293
Klopman G (1964) J Am Chem Soc 86:1463. https://doi.org/10.1021/ja01062a001
Klopman G (1968) J Am Chem Soc 90:223. https://doi.org/10.1021/ja01004a002
Pearson RG (1999) J Chem Edu 76:267. https://doi.org/10.1021/ed076p267
Pearson RG (1986) Proc Natl Acad Sci 83:8440. https://doi.org/10.1073/pnas.83.22.8440
Pearson RG (1988) Inorg Chem 27:734. https://doi.org/10.1021/ic00277a030
Tozer DJ, Proft FD (2005) J Phys Chem A 109:8923. https://doi.org/10.1021/jp053504y
Geerlings P, Proft FD, Langenaeker W (2003) Chem Rev 103:1793. https://doi.org/10.1021/cr990029p
Pearson RG (1987) J Chem Educ 64:561. https://doi.org/10.1021/ed064p561
Chattaraj PK, Sengupta S (1996) J Phys Chem 100:16126. https://doi.org/10.1021/jp961096f
Noorizadeh S (2005) THEOCHEM 713:27. https://doi.org/10.1016/j.theochem.2004.09.029
Kaya S, Kaya C (2015) Mol Phys 113:1311. https://doi.org/10.1080/00268976.2014.991771
Cardenas C, Heidar-Zadeh F, Ayers PW (2016) Phys Chem Chem Phys. 18:25721. https://doi.org/10.1039/C6CP04533B
Reed JL (1997) J Phys Chem A 101:7396. https://doi.org/10.1021/jp9711050
Parr RG, von Szentpaly L, Liu S (1999) J Am Chem Soc 121:1922. https://doi.org/10.1021/ja983494x
Chattaraj PK, Maiti B (2001) J Phys Chem A 105:169. https://doi.org/10.1021/jp0019660
Gordy W (1946) Phys Rev 69:604. https://doi.org/10.1103/PhysRev.69.604
Tandon H, Chakraborty T, Suhag V (2019) Int J Quant Struct Prop Relationsh 4:99. http://sci-hub.tw/10.4018/ijqspr.2019070104
Ghosh DC, Biswas R (2002) Int J Mol Sci 3:87. https://doi.org/10.3390/i3020087
Chakraborty T, Gazi K, Ghosh DC (2010) Mol Phys 108:2081. https://doi.org/10.1080/00268976.2010.505208
Ayers PW, Parr RG (2008). J Chem Phys. https://doi.org/10.1063/1.2957900
Yang W, Lee C, Ghosh SK (1985) J Phys Chem 89:5412. https://doi.org/10.1021/j100271a019
Datta D (1986) J Phys Chem 90:4216. https://doi.org/10.1021/j100408a076
Ghosh DC, Islam N (2011) Int J Quantum Chem 111:1961. https://doi.org/10.1002/qua.22508
Kaya S, Kaya C (2015) Inorg Chem 54:8207. https://doi.org/10.1021/acs.inorgchem.5b00383
Tandon H, Chakraborty T, Suhag V (2019) Chem Biomol Eng 4:45. http://article.sciencepublishinggroup.com/pdf/10.11648.j.cbe.20190404.11.pdf
Tandon H, Chakraborty T, Suhag V (2019) Res Med Eng Sci 7:791. https://crimsonpublishers.com/rmes/pdf/RMES.000668.pdf
Tandon H, Ranjan P, Chakraborty T, Suhag V (2021) Mol Divers 25:249. https://doi.org/10.1007/s11030-020-10062-w
Waddington TC (1959) Adv Inorg Chem Radiochem 1:157. https://doi.org/10.1016/S0065-2792(08)60254-X
Sen KD, Vinayagam SC (1988) Chem Phys Lett 144:178. https://doi.org/10.1016/0009-2614(88)87112-4
Pyykkö P (2012) Annu Rev Phys Chem 63:45. https://doi.org/10.1146/annurev-physchem-032511-143755
Balasubramanian K (1997) Relativistic effects in chemistry: part A theory & techniques. John Wiley & Sons, New York
Balasubramanian K (1997) Relativistic Effects in Chemistry Part B: Applications. Wiley, New York
Thayer JS (2010) Challenges and advances in computational chemistry and physics. In: Barysz M, Ishikawa Y (eds) Relativistic methods for chemists, vol 10. Springer, Dordrecht, pp 63–97
Balasubramanian K, Pitzer KS (1983) J Chem Phys 78:321. https://doi.org/10.1063/1.444504
Balasubramanian K, Sumathi K, Dai D (1991) J Chem Phys 95:3494. https://doi.org/10.1063/1.460852
Pearson RG (1988) Inorg Chem 27:734. https://doi.org/10.1021/ic00277a030
Robles J, Bartolotti LJ (1984) J Am Chem Soc 106:3723. https://doi.org/10.1021/ja00325a003
Szarek P, Grochala W (2014) J Phys Chem A 118:10281. https://doi.org/10.1021/jp507423p
Tandon H, Chakraborty T, Suhag V (2019) J Math Chem 57:2142. https://doi.org/10.1007/s10910-019-01055-8
Pershina V (2014). In: Schädel M, Shaughnessy D (eds) The chemistry of superheavy elements. Springer, Heidelberg, pp 135–239
Balasubramanian K (2001) Chem Phys Lett 341:607. https://doi.org/10.1016/S0009-2614(01)00413-4
Acknowledgements
This manuscript has been prepared for the Special Issue of the Theoretical Chemistry Accounts dedicated to celebrate 80th birth anniversary of renowned Theoretical and Computational Chemist Prof. (Dr.) Ramon Carbó-Dorca. Dr. Tanmoy Chakraborty is thankful to Sharda University, and Dr. Hiteshi Tandon is thankful to Manipal University Jaipur for providing computational resources and research facility.
Funding
Dr. Tanmoy Chakraborty would like to acknowledge the funding support from Science and Engineering Research Board (SERB), Department of Science and Technology, Government of India, under Grant No. [CRG/2020/002951].
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Published as part of the special collection of articles "Festschrift in honour of Prof. Ramon Carbó-Dorca".
Rights and permissions
About this article
Cite this article
Yadav, P., Tandon, H., Malik, B. et al. An alternative approach to compute atomic hardness. Theor Chem Acc 140, 60 (2021). https://doi.org/10.1007/s00214-021-02768-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-021-02768-3