Abstract
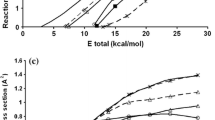
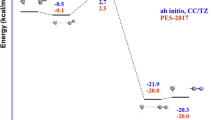
Two important issues were analysed in the title reaction: the effects of vibrational excitation, associated with mode selectivity, and the role of translational energy, associated with Polanyi’s rules. Based on a global analytical potential energy surface, PES-2018, recently developed in our group, quasi-classical trajectory (QCT) calculations were performed at total energy of 35 kcal mol−1, either as translation or as a combination of translation and vibration energy. Independent vibrational excitation by one quantum of any of the CH3 stretching modes in ethane leads to similar dynamics pictures of reaction cross sections and H2(v′, j′) rotovibrational and scattering distributions, ruling out mode selectivity. Normal mode analysis showed a cold, non-inverted, H2(v′) product vibrational distribution, while the C2H5(v′) co-product presented many vibrational states, all of them with a low population, practically simulating a classical behaviour. An equivalent amount of energy as translation raises reactivity somewhat less effective than vibrational energy, contrary to that found for the O(3P) + CH4 reaction. Both reactions present “central” barriers, so this opposite behaviour shows the difficulties for a straightforward application of the Polanyi′s rules. The role of vibrational and translational energy on dynamics has been rationalized by the coupling between vibrational modes, which makes analysis of vibrational excitation difficult in polyatomic systems. Finally, the role of the total energy on reactivity and mode selectivity was analysed, concluding that at lower energy, 15 kcal mol−1, translational energy is much more effective than vibrational energy to enhance reactivity, while at intermediate energy, 20 kcal mol−1, the situation is more confusing and strongly dependent on the counting methods used in the QCT calculations. Therefore, very small mode selectivity is found, and translation seems to be more effective in enhancing reactivity than vibration at low collision energies, while this behaviour is reversed as we increase the collision energy, being the turning point around 20 kcal mol−1.








Similar content being viewed by others
References
Espinosa-Garcia J, Garcia-Chamorro M, Corchado JC (2019) Phys Chem Chem Phys 21:13165
Espinosa-Garcia J, Corchado JC (2019) Phys Chem Chem Phys 21:13305
Polanyi JC (1972) Acc Chem Res 5:161
Corchado JC, Espinosa-Garcia J (2009) Phys Chem Chem Phys 11:10157
Fermi E (1931) Z Phys 71:250
Amat G, Pimbert M (1965) J Mol Spectrosc 16:278
Porter RN, Raff LM (1976) In: Miller WH (ed) Dynamics of molecular collisions, Part B. Plenum Press, New York
Truhlar DG, Muckerman JT (1979) In: Bernstein RB (ed) Atom-molecules collision theory. Plenum Press, New York
Raff LM, Thompson DL (1985) In: Baer M (ed) Theory of chemical reaction dynamics, vol. 3. CRC Press, Boca Raton
Hu X, Hase WL, Pirraglia Y (1991) J Comput Chem 12:1014
Hase WL, Duchovic RJ, Hu X, Komornicki A, Lim KF, Lu D-H, Peslherbe GH, Swamy KN, Van de Linde SR, Varandas AJC, Wang H, Wolf RJ (1996) QCPE Bull. 16:43
Espinosa-Garcia J (2009) J Chem Phys 130:054305
Ping L, Tian L, Song H, Yang M (2018) J Phys Chem A 122:6997
Bonnet L (2013) Int Rev Phys Chem 32:171
Czako G, Bowman JM (2009) J Chem Phys 131:244302
Bonnet L, Espinosa-Garcia J (2010) J Chem Phys 133:164108
Kudla K, Schatz GC (1993) Chem Phys 175:71
Bethardy GA, Wagner AF, Schatz GC, ter Horst MA (1997) J Chem Phys 106:6001
Truhlar DG, Blais NC (1977) J Chem Phys 67:1532
Camden JP, Bechtel HA, Brown DJA, Zare RN (2005) J Chem Phys 123:134301
Jordan MJT, Gilbert RG (1995) J Chem Phys 102:5669
Rudić S, Murray C, Harvey JN, Orr-Ewing AJ (2004) J Chem Phys 120:186
Chakraborty A, Zhao Y, Lin H, Truhlar DG (2006) J Chem Physcs 124:044315
Hu W, Lendvay G, Troya D, Martin MR, Zare RN (2006) J Phys Chem A 110:3017
Layfield JP, Owens MD, Troya D (2008) J Chem Phys 128:94302
Greaves SJ, Orr-Ewing AJ, Troya D (2008) J Phys Chem A 112:9387
Corchado JC, Bravo JL, Espinosa-Garcia J (2009) J Chem Phys 130:184314
Miller WH, Handy NC, Adams JE (1980) J Chem Phys 72:99
Zheng J, Zhang S, Lynch BJ, Corchado JC, Chuang Y-Y, Fast PL, Hu W-P, Liu Y-P, Lynch GC, Nguyen KA, Truhlar DG (2010) POLYRATE-2010-A. University of Minnesota, Minneapolis, MN
Kraka E, Dunning TH (1990) In: Advances in molecular electronic structure theory, vol 1, JAI, New York, p 129
Song H, Li J, Jiang B, Yang M, Lu Y, Guo H (2014) J Chem Phys 140:084307. https://doi.org/10.1063/1.4866426
Jiang B, Guo H (2013) J Am Chem Soc 135:15251
Li J, Guo H (2014) J Phys Chem A 118:2419
Jiang B, Guo H (2013) J Chem Phys 138:234104
Jiang B, Guo H (2014) J Chin Chem Soc 61:847
Kang WK, Jung KW, Kim D-Ch, Jung K-H, Im H-S (1995) Chem Phys 196:363
Zhu Q, Cao JR, Wen Y, Zhang J, Zhang X, Huang Y, Fang W, Wu X (1988) Chem Phys Lett 144:486
Chakravorty KK, Bernstein RB (1984) J Phys Chem 88:3465
Mackay RS, Xu Q-X, Aoiz FJ, Bernstein RB (1991) J Phys Chem 95:8226
Germann GJ, Huh YD, Valentini JJ (1991) Chem Phys Lett 183:353
Germann GJ, Huh YD, Valentini JJ (1992) J Chem Phys 96:1957
Germann GJ, Huh YD, Valentini JJ (1992) J Chem Phys 96:5746
Espinosa-Garcia J, Corchado JC (2015) Theor Chem Acc 134:6
Bonnet L, Corchado JC, Espinosa-Garcia J (2016) C R Chim 19:571
Espinosa-Garcia J, Garcia-Chamorro M (2018) Phys Chem Chem Phys 20:26634
Rangel C, Corchado JC, Espinosa-Garcia J (2006) J Phys Chem A 110:10375
Zhang B, Liu K (2005) J Phys Chem A 109:6791
Acknowledgements
This work was partially supported by Junta de Extremadura and European Regional Development Fund, Spain (Project Nos. GR18010 and IB16013).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Espinosa-Garcia, J., Calle-Cancho, J. & Corchado, J.C. QCT study of the vibrational and translational role in the H + C2H6(ν1, ν2, ν5, ν7, ν9 and ν10) reactions. Theor Chem Acc 138, 116 (2019). https://doi.org/10.1007/s00214-019-2504-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-019-2504-4