Abstract
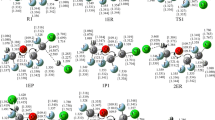
The reaction of CH3OCF2CF2OCHO with Cl atom has been investigated theoretically by direct dynamics method. The BB1K hybrid functional in conjunction with the 6-31 + G(d,p) basis set has been used to optimize the geometries for the stationary points and explore the potential energy surface of the reaction. Four rotation conformers (RC1-4) of CH3OCF2CF2OCHO are identified, and they are all considered in the kinetic calculation. For each conformer, there are two kinds of H-abstraction channels and one displacement channel, and the latter one should be negligible due to involving much higher energy barrier than the former two. The individual rate constants for each H-abstraction channel are evaluated by the improved canonical variational transition-state theory with a small-curvature tunneling correction. The overall rate constant is evaluated by the Boltzmann distribution function, and a fitted four-parameter rate constant expression is obtained over a wide temperature range of 200–2,000 K. The agreement between the calculated and available experimental value at 296 K is good. The contribution of each conformer to the title reaction is discussed with respect to the temperature. In addition, because of the lack of available experimental data for the species involved in the reactions, the enthalpies of the formation (ΔH f,298°) for the reactant and its product radicals are predicted via isodesmic reaction at the BB1K/6-31 + G(d,p) level.





Similar content being viewed by others
References
Marchionni G, Visca M, Eur Pat Appl. 1275678A 2003 (Chem Abs 138, 90675)
Sianesi D, Marchionni G, De Pasquale RJ (1994) In: Banks RE (ed) Organofluorine chemistry: principles and commercial applications. Plenum Press, New York
Marchionni G, Ajroldi G, Pezzin G (1996) In: Agarwal SL, Russom S (eds) Comprehensive polymer science (Second Supplement). Pergamon, London
Marchionni G, Guarda PA (1998) U.S. Patent, 5,744,651
Yu HB, Cui FC, Wang YX, Liu HX, Liu JY (2011) J Theor Comp Chem 10:231–244
Sulback Andersen MP, Hurley MD, Wallington TJ, Blandini F, Jensen NR, Librando V, Hjorth J, Marchionni G, Avataneo M, Visca M, Nicolaisen FM, Nielsen OJ (2004) J Phys Chem A 108:1964–1972
Zhao Y, Lynch BJ, Truhlar DG (2004) J Phys Chem A 108:2715
Truhlar DG, Garrett BC (1980) Acc Chem Res 13:440
Truhlar DG, Isaacson AD, Garrett BC (1985) Generalized transition state theory. In: Baer M (ed) The theory of chemical reaction dynamics, vol 4. CRC Press, Boca Raton, p 65
Truhlar DG, Garrett BC (1984) Annu Rev Phys Chem 35:159
Frisch MJ, Trucks GW, Schlegel HB, Scuseria GE, Robb MA, Cheeseman JR, ScalmaniG, Barone V, Mennucci B, Petersson GA, Nakatsuji H, Caricato M, Li X, Hratchian HP, Izmaylov AF, Bloino J, Zheng G, Sonnenberg JL, Hada M, Ehara M, Toyota K, Fukuda R, Hasegawa J, Ishida M, Nakajima T, Honda Y, Kitao O, Nakai H, Vreven T, Montgomery JA Jr, Peralta JE, Ogliaro F, Bearpark M, Heyd JJ, Brothers E, Kudin KN, Staroverov VN, Kobayashi R, Normand J, Raghavachari K, Rendell A, Burant JC, Iyengar SS, Tomasi J, Cossi M, Rega N, Millam NJ, Klene M, Knox JE, Cross JB, Bakken V, Adamo C, Jaramillo J, Gomperts R, Stratmann RE, Yazyev O, Austin AJ, Cammi R, Pomelli C, Ochterski JW, Martin RL, Morokuma K, Zakrzewski VG, Voth GA, Salvador P, Dannenberg JJ, Dapprich S, Daniels AD, Farkas O, Foresman JB, Ortiz JV, Cioslowski J, Fox DJ (2009) Gaussian 09, revision A.1. Gaussian, Inc., Wallingford, CT
Becke AD (1988) Phys Rev A 38:3098
Becke AD (1996) J Chem Phys 104:1040
Zhao Y, Gonzales-Garcia N, Truhlar DG (2005) J Phys Chem A 109:2012
Parveen S, Chandra AK (2009) J Phys Chem A 113:177–183
Gao H, Wang YX, Liu JY, Yang L, Li ZS, Sun CC (2008) J Phys Chem A 112:4176–4185
Hemelsoet K, Moran D, Speybroeck VV, Waroquier M, Radom L (2006) J Phys Chem A 110:8942
Corchado JC, Chuang YY, Fast PL, Hu WP, Liu YP, Lynch GC, Nguyen KA, Jackels CF, Ramos AF, Ellingson BA, Lynch BJ, Melissas VS, Villa J, Rossi I, Coitino EL, Pu J, Albu TV, Steckler R, Garrett BC, Isaacson AD, Truhlar DG (2007) POLYRATE, version 9.7. University of Minnesota, Minneapolis
Garrett BC, Truhlar DG, Grev RS, Magnuson AW (1980) J Phys Chem 84:1730–1748
Lu DH, Truong TN, Melissas VS, Lynch GC, Liu YP, Grarrett BC, Steckler R, Issacson AD, Rai SN, Hancock GC, Lauderdale JG, Joseph T, Truhlar DG (1992) Comput Phys Commun 71:235
Liu Y-P, Lynch GC, Truong TN, Lu D-H, Truhlar DG, Garrett BC (1993) J Am Chem Soc 115:2408
Piter KS, Gwinn WD (1942) J Chem Phys 10:428
Piter KS (1946) J Chem Phys 14:239
Truhlar DG (1991) J Comput Chem 12:266–270
Chuang YY, Truhlar DG (2000) J Chem Phys 112:1221
Chuang YY, Truhlar DG (2004) J Chem Phys 121:7036
Chuang YY, Truhlar DG (2006) J Chem Phys 124:179903
Ellingson BA, Lynch VA, Mielke SL, Truhlar DG (2006) J Chem Phys 125:084305
Zheng JJ, Truhlar DG (2010) Phys Chem Chem Phys 12:7782–7793
Linstrom PJ, Mallard WG (eds) (2009) Chemistry Webbook NIST. Available from: http://webbook.Nist.Gov/chemistry
Acknowledgments
We thank Professor Donald G. Truhlar for providing the POLYRATE 9.7 program. This work is supported by the National Nature Science Foundation of China (20973077, 20303007) and the Program for New Century Excellent Talents in University (NCET).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Jin, Ty., Yu, Hb., Ci, Cg. et al. Theoretical study for the CH3OCF2CF2OCHO + Cl reaction. Theor Chem Acc 131, 1119 (2012). https://doi.org/10.1007/s00214-012-1119-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00214-012-1119-9