Abstract
Rationale
Substance use disorders are characterized by a loss of executive control over reward-based decision-making, and disruption of fronto-striatal connectivity has been implicated in this process. Sub-anesthetic ketamine has recently been shown to bolster fronto-striatal connectivity in drug-naïve subjects.
Objectives
The influence of ketamine treatment was examined on the disruptive effects of cocaine on functional connectivity (FC) and on cocaine-seeking behavior in female rhesus monkeys.
Methods
Three female rhesus were trained for unanesthetized MRI scanning. Each received three drug-naïve/abstinent pharmacological MRI scans with acute injections of saline, cocaine (0.3 mg/kg i.v.), and cocaine (0.3 mg/kg i.v.) 48-h after a ketamine treatment (low dose = 0.345 mg/kg bolus + 0.256 mg/kg/h for 1 h; i.v.), and a fourth scan with saline injection following 2 months of daily cocaine self-administration. A separate cohort of five rhesus (4 female), all with extensive histories of cocaine exposure, underwent reinstatement testing 48 h after ketamine (or vehicle) treatment. Two sub-anesthetic doses were tested: low dose and high dose = 0.69 mg/kg + 0.512 mg/kg/h for 1 h.
Results
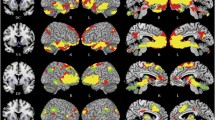
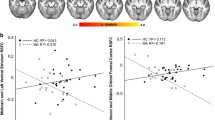
Ketamine treatment attenuated the effects of cocaine on both global and fronto-striatal FC in drug-naïve/abstinent subjects. Two months of daily cocaine self-administration led to prolonged disruption of both global and fronto-striatal FC. Cocaine-seeking behavior during reinstatement was reduced following ketamine treatment at the low dose, but not high dose.
Conclusion
These findings illustrate the disruptive effects of cocaine on functional connectivity and provide evidence for the potential efficacy of ketamine as a treatment for stimulant use disorder.





Similar content being viewed by others
References
aan het Rot M, Collins KA, Murrough JW, Perez AM, Reich DL, Charney DS, Mathew SJ (2010) Safety and efficacy of repeated-dose intravenous ketamine for treatment-resistant depression. Biol Psychiatry 67:139–145
Andersen ML, Sawyer EK, Howell LL (2012) Contributions of neuroimaging to understanding sex differences in cocaine abuse. Exp Clin Psychopharmacol 20:2–15
Anker JJ, Carroll ME (2011) Females are more vulnerable to drug abuse than males: evidence from preclinical studies and the role of ovarian hormones. Curr Top Behav Neurosci 8:73–96
Banks ML, Andersen ML, Murnane KS, Meyer RC, Howell LL (2009) Behavioral and neurochemical effects of cocaine and diphenhydramine combinations in rhesus monkeys. Psychopharmacology 205:467–474
Berman RM, Cappiello A, Anand A, Oren DA, Heninger GR, Charney DS, Krystal JH (2000) Antidepressant effects of ketamine in depressed patients. Biol Psychiatry 47:351–354
Berro LF, Perez Diaz M, Maltbie E, Howell LL (2017) Effects of the serotonin 2C receptor agonist WAY163909 on the abuse-related effects and mesolimbic dopamine neurochemistry induced by abused stimulants in rhesus monkeys. Psychopharmacology (Berl)
Camchong J, Macdonald AW 3rd, Mueller BA, Nelson B, Specker S, Slaymaker V, Lim KO (2014) Changes in resting functional connectivity during abstinence in stimulant use disorder: a preliminary comparison of relapsers and abstainers. Drug Alcohol Depend 139:145–151
Carter LP, Griffiths RR (2009) Principles of laboratory assessment of drug abuse liability and implications for clinical development. Drug Alcohol Depend 105(Suppl 1):S14–S25
Cole MW, Pathak S, Schneider W (2010) Identifying the brain's most globally connected regions. Neuroimage 49:3132–3148
Contreras-Rodriguez O, Albein-Urios N, Perales JC, Martinez-Gonzalez JM, Vilar-Lopez R, Fernandez-Serrano MJ, Lozano-Rojas O, Verdejo-Garcia A (2015) Cocaine-specific neuroplasticity in the ventral striatum network is linked to delay discounting and drug relapse. Addiction 110:1953–1962
Cox RW (1996) AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res 29:162–173
Dakwar E, Hart CL, Levin FR, Nunes EV, Foltin RW (2016) Cocaine self-administration disrupted by the N-methyl-D-aspartate receptor antagonist ketamine: a randomized, crossover trial. Mol Psychiatry
Dakwar E, Levin F, Foltin RW, Nunes EV, Hart CL (2014) The effects of subanesthetic ketamine infusions on motivation to quit and cue-induced craving in cocaine-dependent research volunteers. Biol Psychiatry 76:40–46
Degenhardt L, Baxter AJ, Lee YY, Hall W, Sara GE, Johns N, Flaxman A, Whiteford HA, Vos T (2014) The global epidemiology and burden of psychostimulant dependence: findings from the Global Burden of Disease Study 2010. Drug Alcohol Depend 137:36–47
DePoy LM, Gourley SL (2015) Synaptic cytoskeletal plasticity in the prefrontal cortex following psychostimulant exposure. Traffic 16:919–940
Epstein DH, Preston KL, Stewart J, Shaham Y (2006) Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology 189:1–16
Everitt BJ, Robbins TW (2005) Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nat Neurosci 8:1481–1489
Fitzpatrick CJ, Morrow JD (2017) Subanesthetic ketamine decreases the incentive-motivational value of reward-related cues. J Psychopharmacol 31:67–74
Franceschelli A, Sens J, Herchick S, Thelen C, Pitychoutis PM (2015) Sex differences in the rapid and the sustained antidepressant-like effects of ketamine in stress-naive and “depressed” mice exposed to chronic mild stress. Neuroscience 290:49–60
George O, Koob GF (2010) Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev 35:232–247
Goldstein RZ, Volkow ND (2011) Dysfunction of the prefrontal cortex in addiction: neuroimaging findings and clinical implications. Nat Rev Neurosci 12:652–669
Gopinath K, Maltbie E, Urushino N, Kempf D, Howell L (2016) Ketamine-induced changes in connectivity of functional brain networks in awake female nonhuman primates: a translational functional imaging model. Psychopharmacology 233:3673–3684
Gu H, Salmeron BJ, Ross TJ, Geng X, Zhan W, Stein EA, Yang Y (2010) Mesocorticolimbic circuits are impaired in chronic cocaine users as demonstrated by resting-state functional connectivity. Neuroimage 53:593–601
Haber SN, Knutson B (2010) The reward circuit: linking primate anatomy and human imaging. Neuropsychopharmacology 35:4–26
Hasin DS, O'Brien CP, Auriacombe M, Borges G, Bucholz K, Budney A, Compton WM, Crowley T, Ling W, Petry NM, Schuckit M, Grant BF (2013) DSM-5 criteria for substance use disorders: recommendations and rationale. Am J Psychiatry 170:834–851
Henry PK, Murnane KS, Votaw JR, Howell LL (2010) Acute brain metabolic effects of cocaine in rhesus monkeys with a history of cocaine use. Brain Imaging Behav 4:212–219
Howell LL, Fantegrossi WE (2009) Intravenous drug self-administration in nonhuman primates. In: Buccafusco JJ (ed) Methods of Behavior Analysis in Neuroscience (Frontiers in Neuroscience), Boca Raton (FL)
Ivan Ezquerra-Romano I, Lawn W, Krupitsky E, Morgan CJA (2018) Ketamine for the treatment of addiction: evidence and potential mechanisms. Neuropharmacology 142:72–82
Jentsch JD, Taylor JR (1999) Impulsivity resulting from frontostriatal dysfunction in drug abuse: implications for the control of behavior by reward-related stimuli. Psychopharmacology 146:373–390
Kalivas PW (2009) The glutamate homeostasis hypothesis of addiction. Nat Rev Neurosci 10:561–572
Kalivas PW, O'Brien C (2008) Drug addiction as a pathology of staged neuroplasticity. Neuropsychopharmacology 33:166–180
Kalivas PW, Volkow N, Seamans J (2005) Unmanageable motivation in addiction: a pathology in prefrontal-accumbens glutamate transmission. Neuron 45:647–650
Kalivas PW, Volkow ND (2011) New medications for drug addiction hiding in glutamatergic neuroplasticity. Mol Psychiatry 16:974–986
Kasanetz F, Deroche-Gamonet V, Berson N, Balado E, Lafourcade M, Manzoni O, Piazza PV (2010) Transition to addiction is associated with a persistent impairment in synaptic plasticity. Science 328:1709–1712
Katz JL, Higgins ST (2003) The validity of the reinstatement model of craving and relapse to drug use. Psychopharmacology 168:21–30
Kavalali ET, Monteggia LM (2012) Synaptic mechanisms underlying rapid antidepressant action of ketamine. Am J Psychiatry 169:1150–1156
Koob GF, Volkow ND (2010) Neurocircuitry of addiction. Neuropsychopharmacology 35:217–238
Li N, Lee B, Liu RJ, Banasr M, Dwyer JM, Iwata M, Li XY, Aghajanian G, Duman RS (2010) mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 329:959–964
London ED, Cascella NG, Wong DF, Phillips RL, Dannals RF, Links JM, Herning R, Grayson R, Jaffe JH, Wagner HN Jr (1990) Cocaine-induced reduction of glucose utilization in human brain. A study using positron emission tomography and [fluorine 18]-fluorodeoxyglucose. Arch Gen Psychiatry 47:567–574
Maltbie E, Gopinath K, Urushino N, Kempf D, Howell L (2016) Ketamine-induced brain activation in awake female nonhuman primates: a translational functional imaging model. Psychopharmacology 233:961–972
Maltbie EA, Kaundinya GS, Howell LL (2017) Ketamine and pharmacological imaging: use of functional magnetic resonance imaging to evaluate mechanisms of action. Behav Pharmacol 28:610–622
Martin M, Chen BT, Hopf FW, Bowers MS, Bonci A (2006) Cocaine self-administration selectively abolishes LTD in the core of the nucleus accumbens. Nat Neurosci 9:868–869
McLellan AT, Lewis DC, O'Brien CP, Kleber HD (2000) Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 284:1689–1695
Morgan CJ, Curran HV, Independent Scientific Committee on D (2012) Ketamine use: a review. Addiction 107:27–38
Murnane KS, Gopinath KS, Maltbie E, Daunais JB, Telesford QK, Howell LL (2015) Functional connectivity in frontal-striatal brain networks and cocaine self-administration in female rhesus monkeys. Psychopharmacology 232:745–754
Murnane KS, Howell LL (2010) Development of an apparatus and methodology for conducting functional magnetic resonance imaging (fMRI) with pharmacological stimuli in conscious rhesus monkeys. J Neurosci Methods 191:11–20
National Research Council (U.S.). Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) (2011) Guide for the care and use of laboratory animals. National Academies Press, Washington, D.C., p xxv, 220 p
Perry EB Jr, Cramer JA, Cho HS, Petrakis IL, Karper LP, Genovese A, O'Donnell E, Krystal JH, D'Souza DC, Yale Ketamine Study G (2007) Psychiatric safety of ketamine in psychopharmacology research. Psychopharmacology 192:253–260
Pitts EG, Taylor JR, Gourley SL (2016) Prefrontal cortical BDNF: a regulatory key in cocaine- and food-reinforced behaviors. Neurobiol Dis 91:326–335
Porrino LJ, Smith HR, Nader MA, Beveridge TJ (2007) The effects of cocaine: a shifting target over the course of addiction. Prog Neuro-Psychopharmacol Biol Psychiatry 31:1593–1600
Power JD, Barnes KA, Snyder AZ, Schlaggar BL, Petersen SE (2012) Spurious but systematic correlations in functional connectivity MRI networks arise from subject motion. Neuroimage 59:2142–2154
Preskorn SH, Baker B, Kolluri S, Menniti FS, Krams M, Landen JW (2008) An innovative design to establish proof of concept of the antidepressant effects of the NR2B subunit selective N-methyl-D-aspartate antagonist, CP-101,606, in patients with treatment-refractory major depressive disorder. J Clin Psychopharmacol 28:631–637
Rohlfing T, Kroenke CD, Sullivan EV, Dubach MF, Bowden DM, Grant KA, Pfefferbaum A (2012) The INIA19 template and NeuroMaps atlas for primate brain image parcellation and spatial normalization. Front Neuroinformatics 6:27
RStudio T (2016) RStudio: integrated development environment for R. RStudio, Inc., Boston, MA
Saad ZS, Gotts SJ, Murphy K, Chen G, Jo HJ, Martin A, Cox RW (2012) Trouble at rest: how correlation patterns and group differences become distorted after global signal regression. Brain Connect 2:25–32
Saland SK, Duclot F, Kabbaj M (2017) Integrative analysis of sex differences in the rapid antidepressant effects of ketamine in preclinical models for individualized clinical outcomes. Curr Opin Behav Sci 14:19–26
Sanacora G, Frye MA, McDonald W, Mathew SJ, Turner MS, Schatzberg AF, Summergrad P, Nemeroff CB, American Psychiatric Association Council of Research Task Force on Novel B, Treatments (2017) A consensus statement on the use of ketamine in the treatment of mood disorders. JAMA Psychiatry 74:399–405
Schak KM, Vande Voort JL, Johnson EK, Kung S, Leung JG, Rasmussen KG, Palmer BA, Frye MA (2016) Potential risks of poorly monitored ketamine use in depression treatment. Am J Psychiatry 173:215–218
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM (2004) Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage 23(Suppl 1):S208–S219
Volkow ND, Boyle M (2018) Neuroscience of addiction: relevance to prevention and treatment. Am J Psychiatry 175:729–740
Volkow ND, Hitzemann R, Wang GJ, Fowler JS, Wolf AP, Dewey SL, Handlesman L (1992) Long-term frontal brain metabolic changes in cocaine abusers. Synapse 11:184–190
Volkow ND, Swanson JM (2003) Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry 160:1909–1918
Volkow ND, Tomasi D, Wang GJ, Fowler JS, Telang F, Goldstein RZ, Alia-Klein N, Wong C (2011) Reduced metabolism in brain “control networks” following cocaine-cues exposure in female cocaine abusers. PLoS One 6:e16573
Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C (2008) Dopamine increases in striatum do not elicit craving in cocaine abusers unless they are coupled with cocaine cues. Neuroimage 39:1266–1273
Wan LB, Levitch CF, Perez AM, Brallier JW, Iosifescu DV, Chang LC, Foulkes A, Mathew SJ, Charney DS, Murrough JW (2015) Ketamine safety and tolerability in clinical trials for treatment-resistant depression. J Clin Psychiatry 76:247–252
Wilcox KM, Kimmel HL, Lindsey KP, Votaw JR, Goodman MM, Howell LL (2005) In vivo comparison of the reinforcing and dopamine transporter effects of local anesthetics in rhesus monkeys. Synapse 58:220–228
Yang C, Shirayama Y, Zhang JC, Ren Q, Yao W, Ma M, Dong C, Hashimoto K (2015) R-ketamine: a rapid-onset and sustained antidepressant without psychotomimetic side effects. Transl Psychiatry 5:e632
Zanos P, Moaddel R, Morris PJ, Georgiou P, Fischell J, Elmer GI, Alkondon M, Yuan P, Pribut HJ, Singh NS, Dossou KS, Fang Y, Huang XP, Mayo CL, Wainer IW, Albuquerque EX, Thompson SM, Thomas CJ, Zarate CA Jr, Gould TD (2016) NMDAR inhibition-independent antidepressant actions of ketamine metabolites. Nature 533:481–486
Zarate CA Jr, Brutsche N, Laje G, Luckenbaugh DA, Venkata SL, Ramamoorthy A, Moaddel R, Wainer IW (2012) Relationship of ketamine’s plasma metabolites with response, diagnosis, and side effects in major depression. Biol Psychiatry 72:331–338
Zarate CA Jr, Singh JB, Carlson PJ, Brutsche NE, Ameli R, Luckenbaugh DA, Charney DS, Manji HK (2006) A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch Gen Psychiatry 63:856–864
Zhang JC, Li SX, Hashimoto K (2014) R (−)-ketamine shows greater potency and longer lasting antidepressant effects than S (+)-ketamine. Pharmacol Biochem Behav 116:137–141
Acknowledgements
Special thanks to Dr. Lais Berro and Dr. Maylen Perez-Diaz for behavioral methods training; to Juliet Brown for performing the catheter surgeries; and to technicians Marisa Olsen, Erin Siebert, Ruth Connelly, and Sudeep Patel for laboratory and imaging support.
Funding
This research was supported by P51OD11132 (Yerkes National Primate Research Center) and DA031246 (LLH).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
All protocols and animal care and handling strictly followed the National Institutes of Health Guide for the Care and Use of Laboratory Animals (8th edition, revised 2011) and the recommendations of the American Association for Accreditation of Laboratory Animal Care and were approved by the Institutional Animal Care and Use Committee of Emory University.
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
ESM 1
(DOCX 948 kb)
Rights and permissions
About this article
Cite this article
Maltbie, E.A., Gopinath, K.S. & Howell, L.L. Effects of ketamine treatment on cocaine-induced reinstatement and disruption of functional connectivity in unanesthetized rhesus monkeys. Psychopharmacology 236, 2105–2118 (2019). https://doi.org/10.1007/s00213-019-05204-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-019-05204-4