Abstract
Rationale
Taking high and increasing amounts of cocaine is thought to be necessary for the development of addiction. Consequently, a widely used animal model of drug self-administration involves giving animals continuous drug access during long sessions (LgA), as this produces high and escalating levels of intake. However, human cocaine addicts likely use the drug with an intermittent rather than continuous pattern, producing spiking brain cocaine levels.
Objectives
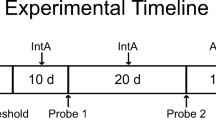
Using an intermittent-access (IntA) cocaine self-administration procedure in rats, we studied the relationship between escalation of cocaine intake and later incentive motivation for the drug, as measured by responding under a progressive ratio schedule of cocaine reinforcement.
Results
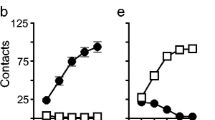
First, under IntA, rats escalated their cocaine use both within and between sessions. However, escalation did not predict later incentive motivation for the drug. Second, incentive motivation for cocaine was similar in IntA-rats limited to low- and non-escalating levels of drug intake (IntA-Lim) and in IntA-rats that took high and escalating levels of drug. Finally, IntA-Lim rats took much less cocaine than rats given continuous drug access during each self-administration session (LgA-rats). However, IntA-Lim rats later responded more for cocaine under a progressive ratio schedule of reinforcement.
Conclusions
Taking large and escalating quantities of cocaine does not appear necessary to increase incentive motivation for the drug. Taking cocaine in an intermittent pattern—even in small amounts—is more effective in producing this addiction-relevant change. Thus, beyond the amount of drug taken, the temporal kinetics of drug use predict change in drug use over time.






Similar content being viewed by others
References
Ahmed SH (2012) The science of making drug-addicted animals. Neuroscience 211:107–125
Ahmed SH, Cador M (2006) Dissociation of psychomotor sensitization from compulsive cocaine consumption. Neuropsychopharmacology 31:563–571
Ahmed SH, Koob GF (1998) Transition from moderate to excessive drug intake: change in hedonic set point. Science 282:298–300
Ahmed SH, Koob GF (1999) Long-lasting increase in the set point for cocaine self-administration after escalation in rats. Psychopharmacology 146:303–312
Allain F, Minogianis EA, Roberts DC, Samaha AN (2015) How fast and how often: the pharmacokinetics of drug use are decisive in addiction. Neurosci Biobehav Rev 56:166–179
Allain F, Roberts DC, Levesque D, Samaha AN (2017) Intermittent intake of rapid cocaine injections promotes robust psychomotor sensitization, increased incentive motivation for the drug and mGlu2/3 receptor dysregulation. Neuropharmacology 117:227–237
APA (2013) DSM V Diagnostic and Statistical Manual of Mental Disorders American Psychiatric Association
Becker JB (2016) Sex differences in addiction. Dialogues Clin Neurosci 18:395–402
Belin D, Balado E, Piazza PV, Deroche-Gamonet V (2009) Pattern of intake and drug craving predict the development of cocaine addiction-like behavior in rats. Biol Psychiatry 65:863–868
Ben-Shahar O, Ahmed SH, Koob GF, Ettenberg A (2004) The transition from controlled to compulsive drug use is associated with a loss of sensitization. Brain Res 995:46–54
Beveridge TJR, Wray P, Brewer A, Shapiro B, Mahoney JJ, Newton TF (2012) Analyzing human cocaine use patterns to inform animal addiction model development. Published abstract for the College on Problems of Drug Dependence annual meeting. Palm Springs, CA
Bouayad-Gervais K, Minogianis EA, Levesque D, Samaha AN (2014) The self-administration of rapidly delivered cocaine promotes increased motivation to take the drug: contributions of prior levels of operant responding and cocaine intake. Psychopharmacology 231:4241–4252
Bozarth MA, Wise RA (1985) Toxicity associated with long-term intravenous heroin and cocaine self-administration in the rat. JAMA 254:81–83
Briand LA, Flagel SB, Garcia-Fuster MJ, Watson SJ, Akil H, Sarter M, Robinson TE (2008) Persistent alterations in cognitive function and prefrontal dopamine D2 receptors following extended, but not limited, access to self-administered cocaine. Neuropsychopharmacology 33:2969–2980
Calipari ES, Ferris MJ, Jones SR (2014a) Extended access of cocaine self-administration results in tolerance to the dopamine-elevating and locomotor-stimulating effects of cocaine. J Neurochem 128:224–232
Calipari ES, Ferris MJ, Siciliano CA, Zimmer BA, Jones SR (2014b) Intermittent cocaine self-administration produces sensitization of stimulant effects at the dopamine transporter. J Pharmacol Exp Ther 349:192–198
Calipari ES, Ferris MJ, Zimmer BA, Roberts DC, Jones SR (2013) Temporal pattern of cocaine intake determines tolerance vs sensitization of cocaine effects at the dopamine transporter. Neuropsychopharmacology 38:2385–2392
Calipari ES, Siciliano CA, Zimmer BA, Jones SR (2015) Brief intermittent cocaine self-administration and abstinence sensitizes cocaine effects on the dopamine transporter and increases drug seeking. Neuropsychopharmacology 40:728–735
Deroche V, Le Moal M, Piazza PV (1999) Cocaine self-administration increases the incentive motivational properties of the drug in rats. Eur J Neurosci 11:2731–2736
Deroche-Gamonet V, Belin D, Piazza PV (2004) Evidence for addiction-like behavior in the rat. Science 305:1014–1017
Edwards S, Koob GF (2013) Escalation of drug self-administration as a hallmark of persistent addiction liability. Behav Pharmacol 24:356–362
Ferrario CR, Gorny G, Crombag HS, Li Y, Kolb B, Robinson TE (2005) Neural and behavioral plasticity associated with the transition from controlled to escalated cocaine use. Biol Psychiatry 58:751–759
Fitch TE, Roberts DC (1993) The effects of dose and access restrictions on the periodicity of cocaine self-administration in the rat. Drug Alcohol Depend 33:119–128
Gawin FH, Kleber HD (1986) Abstinence symptomatology and psychiatric diagnosis in cocaine abusers. Clinical observations. Arch Gen Psychiatry 43:107–113
George O, Mandyam CD, Wee S, Koob GF (2008) Extended access to cocaine self-administration produces long-lasting prefrontal cortex-dependent working memory impairments. Neuropsychopharmacology 33:2474–2482
Hao Y, Martin-Fardon R, Weiss F (2010) Behavioral and functional evidence of metabotropic glutamate receptor 2/3 and metabotropic glutamate receptor 5 dysregulation in cocaine-escalated rats: factor in the transition to dependence. Biol Psychiatry 68:240–248
Hooks MS, Duffy P, Striplin C, Kalivas PW (1994) Behavioral and neurochemical sensitization following cocaine self-administration. Psychopharmacology 115:265–272
Izenwasser S, Cox BM (1990) Daily cocaine treatment produces a persistent reduction of [3H]dopamine uptake in vitro in rat nucleus accumbens but not in striatum. Brain Res 531:338–341
Izenwasser S, Cox BM (1992) Inhibition of dopamine uptake by cocaine and nicotine: tolerance to chronic treatments. Brain Res 573:119–125
Kawa AB, Bentzley BS, Robinson TE (2016) Less is more: prolonged intermittent access cocaine self-administration produces incentive-sensitization and addiction-like behavior. Psychopharmacology 233:3587–3602
Kippin TE, Fuchs RA, See RE (2006) Contributions of prolonged contingent and noncontingent cocaine exposure to enhanced reinstatement of cocaine seeking in rats. Psychopharmacology 187:60–67
Knackstedt LA, Kalivas PW (2007) Extended access to cocaine self-administration enhances drug-primed reinstatement but not behavioral sensitization. J Pharmacol Exp Ther 322:1103–1109
Li DH, Depoortere RY, Emmett-Oglesby MW (1994) Tolerance to the reinforcing effects of cocaine in a progressive ratio paradigm. Psychopharmacology 116:326–332
Martin-Garcia E, Courtin J, Renault P, Fiancette JF, Wurtz H, Simonnet A, Levet F, Herry C, Deroche-Gamonet V (2014) Frequency of cocaine self-administration influences drug seeking in the rat: optogenetic evidence for a role of the prelimbic cortex. Neuropsychopharmacology 39:2317–2330
Minogianis EA, Levesque D, Samaha AN (2013) The speed of cocaine delivery determines the subsequent motivation to self-administer the drug. Neuropsychopharmacology 38:2644–2656
Morgan D, Liu Y, Roberts DC (2006) Rapid and persistent sensitization to the reinforcing effects of cocaine. Neuropsychopharmacology 31:121–128
Morgan D, Smith MA, Roberts DC (2005) Binge self-administration and deprivation produces sensitization to the reinforcing effects of cocaine in rats. Psychopharmacology 178:309–316
Nicola SM, Deadwyler SA (2000) Firing rate of nucleus accumbens neurons is dopamine-dependent and reflects the timing of cocaine-seeking behavior in rats on a progressive ratio schedule of reinforcement. J Neurosci 20:5526–5537
Pan HT, Menacherry S, Justice JB Jr (1991) Differences in the pharmacokinetics of cocaine in naive and cocaine-experienced rats. J Neurochem 56:1299–1306
Paterson NE, Markou A (2003) Increased motivation for self-administered cocaine after escalated cocaine intake. Neuroreport 14:2229–2232
Pitchers KK, Wood TR, Skrzynski CJ, Robinson TE, Sarter M (2017) The ability for cocaine and cocaine-associated cues to compete for attention. Behav Brain Res 320:302–315
Post RM (1980) Intermittent versus continuous stimulation: effect of time interval on the development of sensitization or tolerance. Life Sci 26:1275–1282
Richardson NR, Roberts DC (1996) Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 66:1–11
Roberts DC, Brebner K, Vincler M, Lynch WJ (2002) Patterns of cocaine self-administration in rats produced by various access conditions under a discrete trials procedure. Drug Alcohol Depend 67:291–299
Samaha AN, Li Y, Robinson TE (2002) The rate of intravenous cocaine administration determines susceptibility to sensitization. J Neurosci 22:3244–3250
Samaha AN, Minogianis EA, Nachar W (2011) Cues paired with either rapid or slower self-administered cocaine injections acquire similar conditioned rewarding properties. PLoS One 6:e26481
Shou M, Ferrario CR, Schultz KN, Robinson TE, Kennedy RT (2006) Monitoring dopamine in vivo by microdialysis sampling and on-line CE-laser-induced fluorescence. Anal Chem 78:6717–6725
Siciliano CA, Jones SR (2017) Cocaine potency at the dopamine transporter tracks discrete motivational states during cocaine self-administration. Neuropsychopharmacology 42:1893–1904
Simon SL, Richardson K, Dacey J, Glynn S, Domier CP, Rawson RA, Ling W (2002) A comparison of patterns of methamphetamine and cocaine use. J Addict Dis 21:35–44
Vanderschuren LJ, Everitt BJ (2004) Drug seeking becomes compulsive after prolonged cocaine self-administration. Science 305:1017–1019
Ward AS, Haney M, Fischman MW, Foltin RW (1997) Binge cocaine self-administration in humans: intravenous cocaine. Psychopharmacology 132:375–381
Weeks JR (1962) Experimental morphine addiction: method for automatic intravenous injections in unrestrained rats. Science 138:143–144
Wise RA, Newton P, Leeb K, Burnette B, Pocock D, Justice JB, Jr. (1995) Fluctuations in nucleus accumbens dopamine concentration during intravenous cocaine self-administration in rats. Psychopharmacology 120: 10–20
Zimmer BA, Dobrin CV, Roberts DC (2011) Brain-cocaine concentrations determine the dose self-administered by rats on a novel behaviorally dependent dosing schedule. Neuropsychopharmacology 36:2741–2749
Zimmer BA, Oleson EB, Roberts DC (2012) The motivation to self-administer is increased after a history of spiking brain levels of cocaine. Neuropsychopharmacology 37:1901–1910
Acknowledgments
We are thankful to Dr. David C. S. Roberts for the scientific inspiration leading to this work and to Dr. Terry E. Robinson for wise comments on this manuscript.
Funding
This work was supported by grants to A.N.S from the Canadian Foundation for Innovation (grant number 24326) and the Canadian Institutes of Health Research (grant number 157572). A.N.S is supported by a salary grant from the Fonds de Recherche du Québec—Santé (grant number 28988). F.A is supported by a PhD fellowship from the Groupe de Recherche sur le Système Nerveux Central. A.N.S is a consultant for Nektar Therapeutics.
Author information
Authors and Affiliations
Contributions
F.A performed research and analyzed the data. F.A and A.N.S designed the research and wrote the paper. F.A and K.B.G wrote and tested the computer code needed to apply the IntA drug self-administration procedure to the operant conditioning cages in the laboratory.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Allain, F., Bouayad-Gervais, K. & Samaha, AN. High and escalating levels of cocaine intake are dissociable from subsequent incentive motivation for the drug in rats. Psychopharmacology 235, 317–328 (2018). https://doi.org/10.1007/s00213-017-4773-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4773-8