Abstract
Rationale
Although psilocybin and dextromethorphan (DXM) are hallucinogens, they have different receptor mechanisms of action and have not been directly compared.
Objective
This study compared subjective, behavioral, and physiological effects of psilocybin and dextromethorphan under conditions that minimized expectancy effects.
Methods
Single, acute oral doses of psilocybin (10, 20, 30 mg/70 kg), DXM (400 mg/70 kg), and placebo were administered under double-blind conditions to 20 healthy participants with histories of hallucinogen use. Instructions to participants and staff minimized expectancy effects. Various subjective, behavioral, and physiological effects were assessed after drug administration.
Results
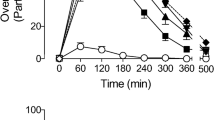
High doses of both drugs produced similar increases in participant ratings of peak overall drug effect strength, with similar times to maximal effect and time-course. Psilocybin produced orderly dose-related increases on most participant-rated subjective measures previously shown sensitive to hallucinogens. DXM produced increases on most of these same measures. However, the high dose of psilocybin produced significantly greater and more diverse visual effects than DXM including greater movement and more frequent, brighter, distinctive, and complex (including textured and kaleidoscopic) images and visions. Compared to DXM, psilocybin also produced significantly greater mystical-type and psychologically insightful experiences and greater absorption in music. In contrast, DXM produced larger effects than psilocybin on measures of disembodiment, nausea/emesis, and light-headedness. Both drugs increased systolic blood pressure, heart rate, and pupil dilation and decreased psychomotor performance and balance.
Conclusions
Psilocybin and DXM produced similar profiles of subjective experiences, with psilocybin producing relatively greater visual, mystical-type, insightful, and musical experiences, and DXM producing greater disembodiment.


Similar content being viewed by others
References
Aghajanian GK, Marek GJ (1999) Serotonin and hallucinogens. Neuropsychopharmacology 21(2 Suppl):16S–23S
Banken JA, Foster H (2008) Dextromethorphan. Ann N Y Acad Sci 1139:402–411
Barrett FS, Griffiths RR (2017) Classic hallucinogens and mystical experiences: phenomenology and neural correlates. Curr Top Behav Neurosci. https://doi.org/10.1007/7854_2017_474
Barrett FS, Johnson MW, Griffiths RR (2015) Validation of the revised mystical experience questionnaire in experimental sessions with psilocybin. J Psychopharmacol 29(11):1182–1190
Barrett FS, Bradstreet MP, Leoutsakos JS, Johnson MW, Griffiths RR (2016) The challenging experience questionnaire: characterization of challenging experiences with psilocybin mushrooms. J Psychopharmacol 30(12):1279–1295
Barrett FS, Robbins H, Smooke D, Brown JL, Griffiths RR (2017). Qualitative and quantitative features of music reported to support peak mystical experiences during psychedelic therapy sessions. Front Psychol 8:1238
Bonny HL, Pahnke WN (1972) The use of music in psychedelic (LSD) psychotherapy. J Music Therapy IX:64–87
Brown RT, Nicholas CR, Cozzi NV, Gassman MC, Cooper KM, Muller D, Thomas CD, Hetzel SJ, Henriquez KM, Ribaudo AS, Hutson PR (2017) Pharmacokinetics of escalating doses of oral psilocybin in healthy adults. Clin Pharmacokinet. https://doi.org/10.1007/s40262-017-0540-6
Carbonaro TM, Eshleman AJ, Forster MJ, Cheng K, Rice KC, Gatch MB (2015) The role of 5-HT2A, 5-HT 2C and mGlu2 receptors in the behavioral effects of tryptamine hallucinogens N,N-dimethyltryptamine and N,N-diisopropyltryptamine in rats and mice. Psychopharmacology 232(1):275–284
Carter LP, Richards BD, Mintzer MZ, Griffiths RR (2006) Relative abuse liability of GHB in humans: a comparison of psychomotor, subjective, and cognitive effects of supratherapeutic doses of triazolam, pentobarbital, and GHB. Neuropsychopharmacology 31(11):2537–2551
Delille HK, Becker JM, Burkhardt S, Bleher B, Terstappen GC, Schmidt M, Meyer AH, Unger L, Marek GJ, Mezler M (2012) Heterocomplex formation of 5-HT2A-mGlu2 and its relevance for cellular signaling cascades. Neuropharmacology 62:2184–2191
Dittrich A (1998) The standardized psychometric assessment of altered states of conciousness (ACSs) in humans. Pharmacopsychiatry 31(Suppl 2):80–84
Fantegrossi WE, Murnane KS, Reissig CJ (2008) The behavioral pharmacology of hallucinogens. Biochem Pharmacol 75(1):17–33
Fribourg M, Moreno JL, Holloway T, Provasi D, Baki L, Mahajan R, Park G, Adnewy SK, Hatcher C, Eltit JM, Ruta JD, Albizu L, Li Z, Umali A, Shim J, Fabiato A, MacKerell AD Jr, Brezina V, Sealfron SC, Filizola M, Gonzalez-Maeso J, Logothetis DE (2011) Decoding the signaling of a GPCR heteromeric complex reveals a unifying mechanism of action of antipsychotic drugs. Cell 147(5):1011–1023
Gonzalez-Maeso J, Weisstaub NV, Zhou M, Chan P, Ivic L, Ang R, Lira A, Bradley-Moore M, Ge Y, Zhou Q, Sealfon SC, Gingrich JA (2007) Hallucinogens recruit specific cortical 5-HT(2A) receptormediated signaling pathways to affect behavior. Neuron 53:439–452
Gonzalez-Maeso J, Ang RL, Yuen T, Chan P, Weisstaub NV, Lopez-Gimenez JF, Zhou M, Okawa Y, Callado LF, Milligan G, Gingrich JA, Filizola M, Meana JJ, Sealfon SC (2008) Identification of a serotonin/glutamate receptor complex implicated in psychosis. Nature 452:93–97
Gouzoulis-Mayfrank E, Heekeren K, Neukirch A, Stoll M, Stock C, Obradovic M, Kovar K-A (2005) Psychological effects of (S)-ketamine and N,N-dimethyltryptamine (DMT): a double blind, cross-over study in healthy volunteers. Pharmacopsychiatry 38:301–311
Griffiths RR, Richards WA, McCann U, Jesse R (2006) Psilocybin can occasion mystical-type experiences having substantial and sustained personal meaning and spiritual significance. Psychopharmacology 187(3):268–283 discussion 284-92
Griffiths RR, Johnson MW, Richards WA, Richards BD, McCann U, Jesse R (2011) Psilocybin occasioned mystical-type experiences: immediate and persisting dose-related effects. Psychopharmacology 218(4):649–665
Hasler F, Bourquin D, Brenneisen R, Bar T, Vollenweider FX (1997) Determinaton of psilocin and 4-hydroxyindole-3-acetic acid in plasma by HPLC-ECD and pharmokinetic profiles of oral and intravenous psilocybin in man. Pharm Acta Helv 72(3):175–184
Hasler F, Grimberg U, Benz MA, Huber T, Vollenweider FX (2004) Acute psychological and physiological effects of psilocybin in healthy humans: a double-blind, placebo-controlled dose-effect study. Psychopharmacology 172(2):145–156
Hood RW, Hill PC, Bernard S (2009) The psychology of religion: an empirical approach. Guilford Press, New York
Jansen KLR (2004) Ketamine: dreams and realities. Multidisciplinary Association for Psychedelic Studies (MAPS), Sarasota, FL
Johnson M, Richards WA, Griffiths RR (2008) Human hallucinogen research: guidelines for safety. J Psychopharmacol 22(6):603–620
Kaelen M, Barrett FS, Roseman L, Lorenz R, Family N, Bolstridge M, Carhart-Harris RL (2015) LSD enhances the emotional response to music. Psychopharmacology 232(19):3607–3614
MacLean KA, Leoutsakos JM, Johnson MW, Griffiths RR (2012) Factor analysis of the mystical experience questionnaire: a study of experiences occasioned by the hallucinogen psilocybin. J Sci Study Relig 51(4):721–737
MacLean KA, Johnson MW, Griffiths RR (2015) Hallucinogens and club drugs. In: Galanter M, Kleber HD, Bradu L (eds) The American Psychiatric Press Textbook of Substance Abuse Treatment, Fifth ed. The American Psychiatric Press, Arlington, VA, pp 209–222
Metzner R, Litwin G, Weil G (1965) The relation of expectation and mood to psilocybin reactions: a questionnaire study. Psychedelic Review 5:3–39
Moreno JL, Holloway T, Albizu L, Sealfon SC, González-Maeso J (2011) Metabotropic glutamate mGlu2 receptor is necessary for the pharmacological and behavioral effects induced by hallucinogenic 5-HT2A receptor agonists. Neurosci Lett 493:76–79
Morris H, Wallach J (2014) From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs. Drug Test Anal 7(5):358–367
Mumford GK, Rush CR, Griffiths RR (1995) Abecarnil and alprazolam in humans: behavioral, subjective and reinforcing effects. J Pharmacol Exp Ther 272(2):570–580
Narby J (1999) The cosmic serpent DNA and the origins of knowledge. Jeremy P. Tarcher/Putnam, New York, NY
Nguyen L, Thomas KL, Lucke-Wold BP, Cavendish JZ, Crowe MS, Matsumoto RR (2016) Dextromethorphan: an update on its utility for neurological and neuropsychiatric disorders. Pharmacol Ther 159:1–22
Nichols DE (2016) Psychedelics. Pharmacol Rev 68:264–355
Passie T, Seifert J, Schneider U, Emrich HM (2002) The pharmacology of psilocybin. Addict Biol 7(4):357–364
Preller KH, Vollenweider FX (2016) Phenomenology, structure, and dynamic of psychedelic states. Curr Top Behav Neurosci. https://doi.org/10.1007/7854_2016_459
Reissig CJ, Carter LP, Johnson MW, Mintzer MZ, Klinedinst MA, Griffiths RR (2012) High doses of dextromethorphan, an NMDA antagonist, produce effects similar to classic hallucinogens. Psychopharmacology 223(1):1–15
Rickli A, Moning OD, Hoener MC, Liechti ME (2016) Receptor interaction profiles of novel psychoactive tryptamines compared with classic hallucinogens. Eur Neuropsychopharmacol 26:1327–1337
SAMHSA (2015) 2014 National Survey on Drug Use and Health: detailed tables. Center for Behavioral Health Statistics and Quality. Substance Abuse and Mental Health Services Administration Rockville, MD
SAMHSA (2016) 2015 National Survey on Drug Use and Health: detailed tables. Center for Behavioral Health Statistics and Quality. Substance Abuse and Mental Health Services Administration Rockville, MD
Schartner MM, Carhart-Harris RL, Barrett AB, Seth AK, Muthukumaraswamy SD (2017) Increased spontaneous MEG signal diversity for psychoactive doses of ketamine, LSD and psilocybin. Sci Rep 7:46421
Schmid B, Bircher J, Preisig R, Kupfer A (1985) Polymorphic dextromethorphan metabolism: co-segregation of oxidative O-demethylation with debrisoquin hydroxylation. Clin Pharmacol Ther 38(6):618–624
Schmidt A, Kometer M, Bachmann R, Seifritz E, Vollenweider F (2013) The NMDA antagonist ketamine and the 5-HT agonist psilocybin produce dissociable effects on structural encoding of emotional face expressions. Psychopharmacol 225:227–239
Shulgin A, Shulgin A (1997) TiHKAL. Transform Press, Berkeley, CA
Strassman R (2001) DMT: the spirit molecule. Park Street Press, Rochester, VA
Strassman RJ, Qualls CR, Uhlenhuth EH, Kellner R (1994) Dose response study of N,N-dimethyltryptamine in humans. II. Subjective effects and preliminary results of a new rating scale. Arch Gen Psychiatry 51:98–108
Studerus E, Gamma A, Vollenweider FX (2010) Psychometric evaluation of the altered states of consciousness rating scale (OAV). PLoS One 5(8):e12412
Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR (2016) Pharmacology of dextromethorphan: relevance to dextromethorphan/quinidine (nuedexta(R)) clinical use. Pharmacol Ther 164:170–182
Tyls F, Palenicek T, Horacek J (2014) Psilocybin—summary of knowledge and new perspectives. Eur Neuropsychopharmacol 24(3):342–356
Vengurlekar SS, Heitkamp J, McCush F, Velagaleti PR, Brisson JH, Bramer SL (2002) J Pharm Biomed Anal 30(1):113–124
Vollenweider FX, Kometer M (2010) The neurobiology of psychedelic drugs: implications for the treatment of mood disorders. Nat Rev Neurosci 11(9):642–651
Vollenweider FX, Vollenweider-Scherpenhuyzen MF, Babler A, Vogel H, Hell D (1998) Psilocybin induces schizophrenia-like psychosis in humans via a serotonin-2 agonist action. Neuroreport 9(17):3897–3902
White W (2002) The DXM experience. https://www.erowid.org/chemicals/dxm/faq/dxm_experience.shtml#toc.5. Accessed 15 July 2017
Winter JC (2009) Hallucinogens as discriminative stimuli in animals: LSD, phenethylamines, and tryptamines. Psychopharmacology 203:251–263
Winter JC, Eckler JR, Rabin RA (2004) Serotonergic/glutamatergic interactions: the effects of mGlu2/3 receptor ligands in rats trained with LSD and PCP as discriminative stimuli. Psychopharmacology 172:233–240
Zentner M, Grandjean D, Scherer KR (2008) Emotions evoked by the sound of music: characterization, classification, and measurement. Emotion 8:494–521
Acknowledgements
We thank Mary Cosimano, M.S.W, Taylor Marcus, Darrick May, M.D., and five other staff members for their roles as session monitors, Dr. Annie Umbricht for medical management, Frederick Barrett, Ph.D., for contributing to the study design, Lisa Schade for technical assistance, Linda Felch for statistical assistance, and the pharmacy and medical staff. We also thank David Nichols, Ph.D., for synthesizing the psilocybin and Michelle Rudek, Pharm.D., Ph.D., and Nichole Anders of the Analytical Pharmacology Core for analyzing dextromethorphan metabolism. The study was conducted in compliance with US laws.
Funding
Conduct of this research was supported by NIH R01DA03889 and T32 DA07209. Support for dextromethorphan metabolic analysis was supported by NIH grants P30CA006973 and UL1TR001079 and the Shared Instrument Grant S10OD020091 to the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
The study was approved by the Institutional Review Board of the Johns Hopkins University School of Medicine. Participants gave their written informed consent before beginning the study procedures and were paid for their participation.
Conflict of interest
Dr. Carbonaro is an employee of the U.S. Food and Drug Administration (FDA); however, the views presented in this article do not necessarily reflect those of the FDA and no official support or endorsement of this article by the FDA is intended or should be inferred. Roland Griffiths is on the Board of Directors of the Heffter Research Institute.
Electronic supplementary material
ESM 1
(PDF 31 kb)
Rights and permissions
About this article
Cite this article
Carbonaro, T.M., Johnson, M.W., Hurwitz, E. et al. Double-blind comparison of the two hallucinogens psilocybin and dextromethorphan: similarities and differences in subjective experiences. Psychopharmacology 235, 521–534 (2018). https://doi.org/10.1007/s00213-017-4769-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4769-4