Abstract
Rationale
Working memory (WM) is a dynamic encoding process and an active representation of information over a short time. The ability to guide forthcoming behavior would be disrupted if WM was impaired by various factors including inflammation, stress, free radicals, and disease states such as schizophrenia. However, the mechanism underlying acute working memory impairment remains to be defined.
Objectives
In this study, we tested the hypothesis that decreased caveolin-1 (Cav-1) and synaptophysin (SYP) accounted for the WM impairment challenged with acute intraperitoneally lipopolysaccharide (LPS), which mimicked neuroinflammation. Delayed alternation T-maze task (DAT) was used to assess working memory of adult male C57BL/6 mice, and western blot and immunostaining were used to detect protein expression and distribution in medial prefrontal cortex (mPFC) and hippocampus.
Results
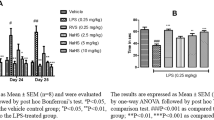
Our results showed that LPS dose-dependently induced working memory deficit accompanied by the decrease of Cav-1 and SYP in mPFC but not hippocampus. In addition, LPS significantly decreased protein level of Cav-1 and SYP in neurons by activating microglia cells. More important, 2-week N-acetylcysteine (NAC) treatment dose-dependently inhibited LPS-induced working memory deficit by improving the ability to use Lose-shift but not Win-shift strategy and significantly inhibited LPS-induced downregulation of Cav-1 and SYP in mPFC.
Conclusions
Taken together, our findings demonstrate that chronic NAC treatment alleviates acute LPS-induced working memory deficit through upregulating Cav-1 and SYP in mice.






Similar content being viewed by others
Abbreviations
- BDL:
-
Bile duct ligation;
- Cav-1:
-
Caveolin-1
- DAT:
-
Delayed alternation T-maze task
- IL-1β:
-
Interleukine-1 beta
- i.p.:
-
Intraperitoneally
- LCM:
-
LPS-conditioned medium
- LPS:
-
lipopolysaccharide
- mPFC:
-
Medial prefrontal cortex
- NAC:
-
N-acetylcysteine
- ROS:
-
Reactive oxygen series
- SYP:
-
Synaptophysin
- TNF-α:
-
Tumor necrosis factor-α
- WM:
-
Working memory
References
Alhadidi Q, Shah ZA (2017) Cofilin mediates LPS-induced microglial cell activation and associated neurotoxicity through activation of NF-kappaB and JAK-STAT pathway. Mol Neurobiol. https://doi.org/10.1007/s12035-017-0432-7
Arai K, Matsuki N, Ikegaya Y, Nishiyama N (2001) Deterioration of spatial learning performances in lipopolysaccharide-treated mice. Jpn J Pharmacol 87:195–201
Arnsten AF (2009) Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci 10:410–422
Baddeley A (2003) Working memory: looking back and looking forward. Nat Rev Neurosci 4:829–839
Cai L, Yi F, Dai Z, Huang X, Zhao YD, Mirza MK, Xu J, Vogel SM, Zhao YY (2014) Loss of caveolin-1 and adiponectin induces severe inflammatory lung injury following LPS challenge through excessive oxidative/nitrative stress. Am J Phys Lung Cell Mol Phys 306:L566–L573
Castner SA, Goldman-Rakic PS, Williams GV (2004) Animal models of working memory: insights for targeting cognitive dysfunction in schizophrenia. Psychopharmacology 174:111–125
Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW (2008) Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain Behav Immun 22:301–311
Costa M, Bernardi J, Fiuza T, Costa L, Brandao R, Pereira ME (2016) N-acetylcysteine protects memory decline induced by streptozotocin in mice. Chem Biol Interact 253:10–17
Cross-Mellor SK, Foley KA, Parker LA, Ossenkopp KP (2009) Lipopolysaccharide dose dependently impairs rapid toxin (LiCl)-induced gustatory conditioning: a taste reactivity examination of the conditioned taste aversion. Brain Behav Immun 23:204–216
Custodio CS, Mello BS, Cordeiro RC, de Araujo FY, Chaves JH, Vasconcelos SM, Nobre Junior HV, de Sousa FC, Vale ML, Carvalho AF, Macedo DS (2013) Time course of the effects of lipopolysaccharide on prepulse inhibition and brain nitrite content in mice. Eur J Pharmacol 713:31–38
Dai XJ, Li N, Yu L, Chen ZY, Hua R, Qin X, Zhang YM (2015) Activation of BV2 microglia by lipopolysaccharide triggers an inflammatory reaction in PC12 cell apoptosis through a toll-like receptor 4-dependent pathway. Cell Stress Chaperones 20:321–331
Dehkordi NG, Noorbakhshnia M, Ghaedi K, Esmaeili A, Dabaghi M (2015) Omega-3 fatty acids prevent LPS-induced passive avoidance learning and memory and CaMKII-alpha gene expression impairments in hippocampus of rat. Pharmacol Rep 67:370–375
Deng XH, Ai WM, Lei DL, Luo XG, Yan XX, Li Z (2012) Lipopolysaccharide induces paired immunoglobulin-like receptor B (PirB) expression, synaptic alteration, and learning-memory deficit in rats. Neuroscience 209:161–170
Dhanda S, Kaur S, Sandhir R (2013) Preventive effect of N-acetyl-L-cysteine on oxidative stress and cognitive impairment in hepatic encephalopathy following bile duct ligation. Free Radic Biol Med 56:204–215
Farr SA, Poon HF, Dogrukol-Ak D, Drake J, Banks WA, Eyerman E, Butterfield DA, Morley JE (2003) The antioxidants alpha-lipoic acid and N-acetylcysteine reverse memory impairment and brain oxidative stress in aged SAMP8 mice. J Neurochem 84:1173–1183
Fu AL, Dong ZH, Sun MJ (2006) Protective effect of N-acetyl-L-cysteine on amyloid beta-peptide-induced learning and memory deficits in mice. Brain Res 1109:201–206
Garrean S, Gao XP, Brovkovych V, Shimizu J, Zhao YY, Vogel SM, Malik AB (2006) Caveolin-1 regulates NF-kappaB activation and lung inflammatory response to sepsis induced by lipopolysaccharide. J Immunol 177:4853–4860
Gaudreault SB, Dea D, Poirier J (2004) Increased caveolin-1 expression in Alzheimer’s disease brain. Neurobiol Aging 25:753–759
Gaudreault SB, Blain JF, Gratton JP, Poirier J (2005) A role for caveolin-1 in post-injury reactive neuronal plasticity. J Neurochem 92:831–839
Gioiosa L, Raggi C, Ricceri L, Jasmin JF, Frank PG, Capozza F, Lisanti MP, Alleva E, Sargiacomo M, Laviola G (2008) Altered emotionality, spatial memory and cholinergic function in caveolin-1 knock-out mice. Behav Brain Res 188:255–262
Goel R, Bhat SA, Hanif K, Nath C, Shukla R (2016) Perindopril attenuates lipopolysaccharide-induced amyloidogenesis and memory impairment by suppression of oxidative stress and RAGE activation. ACS Chem Neurosci 7:206–217
Goldman-Rakic PS (1995) Cellular basis of working memory. Neuron 14:477–485
Goldman-Rakic PS (1996) Regional and cellular fractionation of working memory. Proc Natl Acad Sci U S A 93:13473–13480
Goncalves JF, Fiorenza AM, Spanevello RM, Mazzanti CM, Bochi GV, Antes FG, Stefanello N, Rubin MA, Dressler VL, Morsch VM, Schetinger MR (2010) N-acetylcysteine prevents memory deficits, the decrease in acetylcholinesterase activity and oxidative stress in rats exposed to cadmium. Chem Biol Interact 186:53–60
Hoffer ME, Balaban C, Slade MD, Tsao JW, Hoffer B (2013) Amelioration of acute sequelae of blast induced mild traumatic brain injury by N-acetyl cysteine: a double-blind, placebo controlled study. PLoS One 8:e54163
Jain S, Kumar CH, Suranagi UD, Mediratta PK (2011) Protective effect of N-acetylcysteine on bisphenol A-induced cognitive dysfunction and oxidative stress in rats. Food Chem Toxicol 49:1404–1409
Jatana M, Singh I, Singh AK, Jenkins D (2006) Combination of systemic hypothermia and N-acetylcysteine attenuates hypoxic-ischemic brain injury in neonatal rats. Pediatr Res 59:684–689
Jayalakshmi K, Singh SB, Kalpana B, Sairam M, Muthuraju S, Ilavazhagan G (2007) N-acetyl cysteine supplementation prevents impairment of spatial working memory functions in rats following exposure to hypobaric hypoxia. Physiol Behav 92:643–650
Jin X, Sun Y, Xu J, Liu W (2015) Caveolin-1 mediates tissue plasminogen activator-induced MMP-9 up-regulation in cultured brain microvascular endothelial cells. J Neurochem 132:724–730
Kim JR, Ryu HH, Chung HJ, Lee JH, Kim SW, Kwun WH, Baek SH, Kim JH (2006) Association of anti-obesity activity of N-acetylcysteine with metallothionein-II down-regulation. Exp Mol Med 38:162–172
Kirova AM, Bays RB, Lagalwar S (2015) Working memory and executive function decline across normal aging, mild cognitive impairment, and Alzheimer’s disease. Biomed Res Int 2015:748212
Ku Y, Bodner M, Zhou YD (2015) Prefrontal cortex and sensory cortices during working memory: quantity and quality. Neurosci Bull 31:175–182
Li BM, Funahashi S (2015) A step forward in the understanding of prefrontal cortical functions. Neurosci Bull 31:161–163
Liu J, Jin X, Liu KJ, Liu W (2012) Matrix metalloproteinase-2-mediated occludin degradation and caveolin-1-mediated claudin-5 redistribution contribute to blood-brain barrier damage in early ischemic stroke stage. J Neurosci 32:3044–3057
Liu Y, Liang Z, Liu J, Zou W, Li X, Wang Y, An L (2013) Downregulation of caveolin-1 contributes to the synaptic plasticity deficit in the hippocampus of aged rats. Neural Regen Res 8:2725–2733
Liu Y, Liu WC, Sun Y, Shen X, Wang X, Shu H, Pan R, Liu CF, Liu W, Liu KJ, Jin X (2017) Normobarich extends neuro- and vaso-protection of N-acetylcysteine in transient focal ischemia. Mol Neurobiol 54:3418–3427
Mandyam CD, Schilling JM, Cui W, Egawa J, Niesman IR, Kellerhals SE, Staples MC, Busija AR, Risbrough VB, Posadas E, Grogman GC, Chang JW, Roth DM, Patel PM, Patel HH, Head BP (2015) Neuron-targeted caveolin-1 improves molecular signaling, plasticity, and behavior dependent on the hippocampus in adult and aged mice. Biol Psychiatry 81(2):101–110
Martinez G, Di Giacomo C, Carnazza ML, Sorrenti V, Castana R, Barcellona ML, Perez-Polo JR, Vanella A (1997) MAP2, synaptophysin immunostaining in rat brain and behavioral modifications after cerebral postischemic reperfusion. Dev Neurosci 19:457–464
Martinez M, Hernandez AI, Martinez N (2000) N-acetylcysteine delays age-associated memory impairment in mice: role in synaptic mitochondria. Brain Res 855:100–106
Mohammadi F, Rahimian R, Fakhraei N, Rezayat SM, Javadi-Paydar M, Dehpour AR, Afshari K, Ejtemaei Mehr S (2016) Effect of glatiramer acetate on short-term memory impairment induced by lipopolysaccharide in male mice. Fundam Clin Pharmacol 30:347–356
Murray C, Sanderson DJ, Barkus C, Deacon RM, Rawlins JN, Bannerman DM, Cunningham C (2012) Systemic inflammation induces acute working memory deficits in the primed brain: relevance for delirium. Neurobiol Aging 33(603–616):e3
Otte DM, Sommersberg B, Kudin A, Guerrero C, Albayram O, Filiou MD, Frisch P, Yilmaz O, Drews E, Turck CW, Bilkei-Gorzo A, Kunz WS, Beck H, Zimmer A (2011) N-acetyl cysteine treatment rescues cognitive deficits induced by mitochondrial dysfunction in G72/G30 transgenic mice. Neuropsychopharmacology 36:2233–2243
Pan XD, Chen XC, Zhu YG, Zhang J, Huang TW, Chen LM, Ye QY, Huang HP (2008) Neuroprotective role of tripchlorolide on inflammatory neurotoxicity induced by lipopolysaccharide-activated microglia. Biochem Pharmacol 76:362–372
Prakash A, Kalra JK, Kumar A (2015) Neuroprotective effect of N-acetyl cysteine against streptozotocin-induced memory dysfunction and oxidative damage in rats. J Basic Clin Physiol Pharmacol 26:13–23
Rodrigues FS, Souza MA, Magni DV, Ferreira AP, Mota BC, Cardoso AM, Paim M, Xavier LL, Ferreira J, Schetinger MR, Da Costa JC, Royes LF, Fighera MR (2013) N-acetylcysteine prevents spatial memory impairment induced by chronic early postnatal glutaric acid and lipopolysaccharide in rat pups. PLoS One 8:e78332
Russelakis-Carneiro M, Hetz C, Maundrell K, Soto C (2004) Prion replication alters the distribution of synaptophysin and caveolin 1 in neuronal lipid rafts. Am J Pathol 165:1839–1848
Schwabe K, Enkel T, Klein S, Schutte M, Koch M (2004) Effects of neonatal lesions of the medial prefrontal cortex on adult rat behaviour. Behav Brain Res 153:21–34
Shu H, Zheng GQ, Wang X, Sun Y, Liu Y, Weaver JM, Shen X, Liu W, Jin X (2015) Activation of matrix metalloproteinase in dorsal hippocampus drives improvement in spatial working memory after intra-VTA nicotine infusion in rats. J Neurochem 135:357–367
Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR (2000) Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci 20:6587–6593
Song X, Zhou B, Zhang P, Lei D, Wang Y, Yao G, Hayashi T, Xia M, Tashiro S, Onodera S, Ikejima T (2016) Protective effect of silibinin on learning and memory impairment in LPS-treated rats via ROS-BDNF-TrkB pathway. Neurochem Res 41:1662–1672
Sparkman NL, Buchanan JB, Heyen JR, Chen J, Beverly JL, Johnson RW (2006) Interleukin-6 facilitates lipopolysaccharide-induced disruption in working memory and expression of other proinflammatory cytokines in hippocampal neuronal cell layers. J Neurosci 26:10709–10716
Takayasu Y, Takeuchi K, Kumari R, Bennett MV, Zukin RS, Francesconi A (2010) Caveolin-1 knockout mice exhibit impaired induction of mGluR-dependent long-term depression at CA3-CA1 synapses. Proc Natl Acad Sci U S A 107:21778–21783
Viana AF, Maciel IS, Dornelles FN, Figueiredo CP, Siqueira JM, Campos MM, Calixto JB (2010) Kinin B1 receptors mediate depression-like behavior response in stressed mice treated with systemic E. coli lipopolysaccharide. J Neuroinflammation 7:98
Walker AK, Budac DP, Bisulco S, Lee AW, Smith RA, Beenders B, Kelley KW, Dantzer R (2013) NMDA receptor blockade by ketamine abrogates lipopolysaccharide-induced depressive-like behavior in C57BL/6J mice. Neuropsychopharmacology 38:1609–1616
Wang GW, Cai JX (2006) Disconnection of the hippocampal-prefrontal cortical circuits impairs spatial working memory performance in rats. Behav Brain Res 175:329–336
Wang X, Xue GX, Liu WC, Shu H, Wang M, Sun Y, Liu X, Sun YE, Liu CF, Liu J, Liu W, Jin X (2017) Melatonin alleviates lipopolysaccharide-compromised integrity of blood-brain barrier through activating AMP-activated protein kinase in old mice. Aging Cell 16:414–421
Wright DJ, Renoir T, Smith ZM, Frazier AE, Francis PS, Thorburn DR, McGee SL, Hannan AJ, Gray LJ (2015) N-acetylcysteine improves mitochondrial function and ameliorates behavioral deficits in the R6/1 mouse model of Huntington's disease. Transl Psychiatry 5:e492
Yoon T, Okada J, Jung MW, Kim JJ (2008) Prefrontal cortex and hippocampus subserve different components of working memory in rats. Learn Mem 15:97–105
Zhang XH, Liu SS, Yi F, Zhuo M, Li BM (2013) Delay-dependent impairment of spatial working memory with inhibition of NR2B-containing NMDA receptors in hippocampal CA1 region of rats. Mol Brain 6:13
Zhou T, Zhao L, Zhan R, He Q, Tong Y, Tian X, Wang H, Zhang T, Fu Y, Sun Y, Xu F, Guo X, Fan D, Han H, Chui D (2014a) Blood-brain barrier dysfunction in mice induced by lipopolysaccharide is attenuated by dapsone. Biochem Biophys Res Commun 453:419–424
Zhou X, Cao Y, Ao G, Hu L, Liu H, Wu J, Wang X, Jin M, Zheng S, Zhen X, Alkayed NJ, Jia J, Cheng J (2014b) CaMKKbeta-dependent activation of AMP-activated protein kinase is critical to suppressive effects of hydrogen sulfide on neuroinflammation. Antioxid Redox Signal 21:1741–1758
Funding
This work was supported, by the National Natural Science Foundation of China (81701316, 81371224, 81671145), by the Natural Science Foundation of Jiangsu Province of China (L221506415, BK20140366), and by the Open Research Fund of State Key Laboratory of Bioelectronics, Southeast University (No. 7). This work was also partly supported by Priority Academic Program Development of Jiangsu Higher Education Institutions of China.
Author information
Authors and Affiliations
Contributions
This work was performed and accomplished by all authors. XS, YS, MW, HS, LJZ, and PY contributed to the execution of the entire research project and the statistical analyses. XS, JZ, and XJ wrote the manuscript. All authors have read and approved the final manuscript.
Corresponding authors
Ethics declarations
All experimental procedures were approved by the University Committee on Animal Care of Soochow University and performed according to the NIH Guide for the Care and Use of Laboratory Animals.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Shen, X., Sun, Y., Wang, M. et al. Chronic N-acetylcysteine treatment alleviates acute lipopolysaccharide-induced working memory deficit through upregulating caveolin-1 and synaptophysin in mice. Psychopharmacology 235, 179–191 (2018). https://doi.org/10.1007/s00213-017-4762-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-017-4762-y