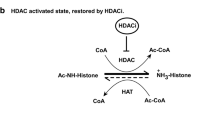
Abstract
Rationale
Aberrations in cellular acetate-utilization processes leading to global histone hypoacetylation have been implicated in the etiology of neuropsychiatric disorders like schizophrenia.
Objectives
Here, we investigated the role of acetate supplementation in the form of glyceryl triacetate (GTA) for the ability to restore the N-methyl D-aspartate (NMDA) receptor-induced histone hypoacetylation and to ameliorate associated behavioral phenotypes in mice.
Results
Taking cues from the studies in SH-SY5Y cells, we monitored acetylation status of specific lysine residues of histones H3 and H4 (H3K9 and H4K8) to determine the impact of oral GTA supplementation in vivo. Mice treated chronically with MK-801 (10 days; 0.15 mg/kg daily) induced hypoacetylation of H3K9 and H4K8 in the hippocampus. Daily oral supplementation of GTA (2.9 g/kg) was able to prevent this MK801-induced hypoacetylation significantly. Though MK-801-stimulated decreases in acetyl-H3K9 and acetyl-H4K8 were found to be associated with ERK1/2 activation, GTA seemed to act independent of this pathway. Simultaneously, GTA administration was able to attenuate the chronic MK-801-induced cognitive behavior phenotypes in elevated plus maze and novel object recognition tests. Not only MK-801, GTA also demonstrated protective effects against behavioral phenotypes generated by another NMDA receptor antagonist, ketamine. Acute (single injection) ketamine-mediated hyperactivity phenotype and chronic (10 days treatment) ketamine-induced phenotype of exaggerated immobility in forced swim test were ameliorated by GTA.
Conclusion
The signature behavioral phenotypes induced by acute and chronic regimen of NMDA receptor antagonists seemed to be attenuated by GTA. This study thus provides a therapeutic paradigm of using dietary acetate supplement in psychiatric disorders.





Similar content being viewed by others
References
Arun P, Madhavarao CN, Moffett JR, Hamilton K, Grunberg NE, Ariyannur PS et al (2010) Metabolic acetate therapy improves phenotype in the tremor rat model of Canavan disease. J Inherit Metab Dis 33:195–210
Cha DS, Kudlow PA, Baskaran A, Mansur RB, McIntyre RS (2014) Implications of epigenetic modulation for novel treatment approaches in patients with schizophrenia. Neuropharmacology 77:481–486
Chatterjee M, Ganguly S, Srivastava M, Palit G (2011) Effect of ‘chronic’ versus ‘acute’ ketamine administration and its ‘withdrawal’ effect on behavioural alterations in mice: implications for experimental psychosis. Behav Brain Res 216:247–254
Chatterjee M, Verma R, Ganguly S, Palit G (2012) Neurochemical and molecular characterization of ketamine-induced experimental psychosis model in mice. Neuropharmacology 63:1161–1171
Choudhury A, Singh S, Palit G, Shukla S, Ganguly S (2015) Administration of N-acetylserotonin and melatonin alleviate chronic ketamine-induced behavioural phenotype accompanying BDNF-independent and dependent converging cytoprotective mechanisms in the hippocampus. Behav Brain Res. doi:10.1016/S0166-4328(15)30241-2.
Crosio C, Heitz E, Allis CD, Borrelli E, Sassone-Corsi P (2003) Chromatin remodeling and neuronal response: multiple signaling pathways induce specific histone H3 modifications and early gene expression in hippocampal neurons. J Cell Sci 116:4905–4914
Day J, Sweatt JD (2011) Epigenetic mechanisms in cognition. Neuron 70:813–829
Fang Y, Iu CY, Lui CN, Zou Y, Fung CK., Li HW, Xi N, Yung KK, Lai KW (2014) Investigating dynamic structural and mechanical changes of neuroblastoma cells associated with glutamate-mediated neurodegeneration. Sci Rep 4:7074. doi:10.1038/srep07074.
Fischer A, Sananbenesi F. Wang X, Dobbin M, Tsai L.-H (2007) Recovery of learning and memory is associated with chromatin remodelling Nature 447:178–182
Fiume M Z, CIRE Panel (2003) Final report on the safety assessment of triacetin. Int J Toxicol 22:1–10
Grüter T, Wiescholleck V, Dubovyk V, Aliane V, Manahan-Vaughan D (2015) Altered neuronal excitability underlies impaired hippocampal function in an animal model of psychosis. Front Behav Neurosci 9: 117. doi:10.3389/fnbeh.2015.00117.
Hasan A, Mitchell A, Schneider A, Halene T, Akbarian S (2013) Epigenetic dysregulation in schizophrenia: molecular and clinical aspects of histone deacetylase inhibitors. Eur Arch Psychiatry Clin Neurosci 263:273–284
Hlinák Z, Krejcí I (2002) MK-801 induced amnesia for the elevated plus-maze in mice. Behav Brain Res 131:221–225
Irifune M, Shimizu T, Nomoto M, Fukuda T (1995) Involvement of N-methyl-D-aspartate (NMDA) receptors in noncompetitive NMDA receptor antagonist-induced hyperlocomotion in mice. Pharmacol Biochem Behav 51:291–296
Itoh J, Nabeshima T, Kameyama T (1990) Utility of an elevated plus-maze for the evaluation of memory in mice: effects of nootropics, scopolamine and electroconvulsive shock. Psychopharmacology (Berl) 101:27–33
Javitt DC (2012) Twenty-five years of glutamate in schizophrenia: are we there yet? Schizophr Bull 38:911–913
Kondziella D, Brenner E, Eyjolfsson EM, Sonnewald U (2007) How do glial-neuronal interactions fit into current neurotransmitter hypotheses of schizophrenia. Neurochem Int 50:291–301
Kurita M, Holloway T, García-Bea A, Kozlenkov A, Friedman AK, Moreno JL et al (2012) HDAC2 regulates atypical antipsychotic responses through the modulation of mGlu2 promoter activity. Nat Neurosci 15:1245–1254
Levenson JM, O’Riordan KJ, Brown KD, Trinh MA, Molfese DL, Sweatt JD (2004) Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem 279:40545–40559
Long PM, Tighe SW, Driscoll HE, Moffett JR, Namboodiri AM, Viapiano MS et al (2013) Acetate supplementation induces growth arrest of NG2/PDGFRα-positive oligodendroglioma-derived tumor-initiating cells. PLoS One 8, e80714
Manahan-Vaughan D, von Haebler D, Winter C, Juckel G, Heinemann U (2008) A single application of MK-801 causes symptoms of acute psychosis, deficits in spatial memory, and impairment of synaptic plasticity in rats. Hippocampus 18:125–134
Martins-de-Souza D, Harris LW, Guest PC, Bahn S (2011) The role of energy metabolism dysfunction and oxidative stress in schizophrenia revealed by proteomics. Antioxid Redox Signal 15:2067–2079
Mathew R, Arun P, Madhavarao CN, Moffett JR, Namboodiri MA (2005) Progress toward acetate supplementation therapy for Canavan disease: glyceryl triacetate administration increases acetate, but not N-acetylaspartate, levels in brain. J Pharmacol Exp Ther 315:297–303
McQuown SC, Barrett RM, Matheos DP, Post RJ, Rogge GA, Alenghat T et al (2011) HDAC3 is a critical negative regulator of long-term memory formation. J Neurosci 31:764–774
Moffett JR, Ross B, Arun P, Madhavarao CN, Namboodiri AM (2007) N-Acetylaspartate in the CNS: from neurodiagnostics to neurobiology. Prog Neurobiol 81:89–131
Morris MJ, Mahgoub M, Na ES, Pranav H, Monteggia LM (2013) Loss of histone deacetylase 2 improves working memory and accelerates extinction learning. J Neurosci 33:6401–6411
Orzelska J, Talarek S, Listos J, Fidecka S (2013) Effects of NOS inhibitors on the benzodiazepines-induced memory impairment of mice in the modified elevated plus-maze task. Behav Brain Res 244:100–106
Peixoto L, Abel T (2013) The role of histone acetylation in memory formation and cognitive impairments. Neuropsychopharmacology 38:62–76
Porsolt RD, Anton G, Blavet N, Jalfre M (1978) Behavioural despair in rats: a new model sensitive to antidepressant treatments. Eur J Pharmacol 47:379–391
Rajagopal L, Massey BW, Huang M, Oyamada Y, Meltzer HY (2014) The novel object recognition test in rodents in relation to cognitive impairment in schizophrenia. Curr Pharm Des 20:5104–5514
Reisenauer CJ, Bhatt DP, Mitteness DJ, Slanczka ER, Gienger HM, Watt JA et al (2011) Acetate supplementation attenuates lipopolysaccharide-induced neuroinflammation. J Neurochem 117:264–274
Rodgers RJ, Johnson NJ, Carr J, Hodgson TP (1997) Resistance of experientially-induced changes in murine plus-maze behaviour to altered retest conditions. Behav Brain Res 86:71–77
Rumbaugh G, Miller CA (2011) Epigenetic changes in the brain: measuring global histone modifications. Methods Mol Biol 670:263–274
Schwerk A, Alves FD, Pouwels PJ, van Amelsvoort T (2014) Metabolic alterations associated with schizophrenia: a critical evaluation of proton magnetic resonance spectroscopy studies. J Neurochem 128:1–87
Segel R, Anikster Y, Zevin S, Steinberg A, Gahl WA (2011) A safety trial of high dose glyceryl triacetate for Canavan disease. Mol Genet Metab 103:203–206
Sharma RP, Grayson DR, Gavin DP (2008) Histone deactylase 1 expression is increased in the prefrontal cortex of schizophrenia subjects: analysis of the National Brain Databank microarray collection. Schizophr Res 98:111–117
Soliman ML, Rosenberger TA (2011) Acetate supplementation increases brain histone acetylation and inhibits histone deacetylase activity and expression. Mol Cell Biochem 352:173–180
Soliman ML, Smith MD, Houdek HM, Rosenberger TA (2012) Acetate supplementation modulates brain histone acetylation and decreases interleukin-1β expression in a rat model of neuroinflammation. J Neuroinflammation 9:51
Soliman ML, Ohm JE, Rosenberger TA (2013) Acetate reduces PGE2 release and modulates phospholipase and cyclooxygenase levels in neuroglia stimulated with lipopolysaccharide. Lipids 48:651–662
Sun L, Li J, Zhou K, Zhang M, Yang J, Li Y et al (2013) Metabolomic analysis reveals metabolic disturbance in the cortex and hippocampus of subchronic MK-801 treated rats. PLoS One 8, e60598
Tsen AR, Long PM, Driscoll HE, Davies MT, Teasdale BA, Penar PL et al (2014) Triacetin-based acetate supplementation as a chemotherapeutic adjuvant therapy in glioma. Int J Cancer 134:1300–1310
Volmar CH, Wahlestedt C (2015) Histone deacetylases (HDACs) and brain function. Neuroepigenetics 1:20–27
Wang CX, Song JH, Song DK, Yong VW, Shuaib A, Hao C (2006) Cyclin-dependent kinase-5 prevents neuronal apoptosis through ERK-mediated upregulation of Bcl-2. Cell Death Differ 13:1203–1212
Warburton EC, Barker GR, Brown MW (2013) Investigations into the involvement of NMDA mechanisms in recognition memory. Neuropharmacology 74:41–47
Zhao Z, Fan L, Fortress AM, Boulware MI, Frick KM (2012) Hippocampal histone acetylation regulates object recognition and the estradiol-induced enhancement of object recognition. J Neurosci 32:2344–2351
Acknowledgments
SG was supported with funds (Grant # BT/PR11062/MED/30/124/2008), from DBT, India, for this study. CSIR Network Project grant miND (BSC0115) to S. Shukla is also thankfully acknowledged. Authors also acknowledge the Council of Scientific & Industrial Research (CSIR) for providing fellowships to S. Singh and PG. We also acknowledge enthusiastic technical support for the animal behavior experiments by Mr. Shubhranshu S. Jena. We sincerely thank Dr. M. A. Namboodiri, Uniformed Services University of the Health Sciences, MD, USA, for his insightful discussions.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Authors have no competing financial interests in relation to the work described.
Additional information
Seema Singh, Arnab Choudhury and Priya Gusain contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 219 kb)
Rights and permissions
About this article
Cite this article
Singh, S., Choudhury, A., Gusain, P. et al. Oral acetate supplementation attenuates N-methyl D-aspartate receptor hypofunction-induced behavioral phenotypes accompanied by restoration of acetyl-histone homeostasis. Psychopharmacology 233, 1257–1268 (2016). https://doi.org/10.1007/s00213-016-4213-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00213-016-4213-1