Abstract
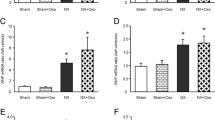
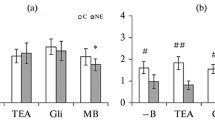
Diabetic nephropathy is associated with increased risk of cardiovascular disease. B-type natriuretic peptide (BNP) plays an important role in cardiovascular pathophysiology and therapeutics. The aim of the present study was to investigate the influence of experimental diabetes on the mechanisms that regulate the relaxant response of the rabbit renal artery to BNP. Arterial relaxations to BNP were enhanced in diabetic rabbits. Indomethacin enhanced BNP-induced relaxation in control rabbits but showed no effect in diabetic rabbits. BNP-induced release of thromboxane A2 or prostacyclin was not different in both groups of animals. Iberiotoxin had no effect on relaxations to BNP in both groups of animals. Charybdotoxin displaced to the right the concentration-response curve to BNP in both group of animals, and inhibited BNP-induced relaxation only in diabetic rabbits. Glibenclamide did not modify the BNP-induced relaxations in control rabbits, but inhibited it in diabetic rabbits. These results suggest that diabetes induces hypereactivity of the rabbit renal artery to BNP by mechanisms that at least include (1) a reduced vasoconstrictor influence of arachidonic acid metabolites via cyclooxygenase 2, which is not related with changes in thromboxane A2 and prostacyclin release from the arterial wall and (2) a selectively increased modulatory activity of KATP and endothelial IKCa channels.









Similar content being viewed by others
References
Carmines PK (2010) The renal vascular response to diabetes. Curr Opin Nephrol Hypertens 19:85–90
Carmines PK, Fujiwara K (2002) Altered electromechanical coupling in the renal microvasculature during the early stage of diabetes mellitus. Clin Exp Pharmacol Physiol 29:143–148
Centeno JM, Marrachelli VG, Miranda L, Castelló-Ruiz M, Burguete MC, Jover-Mengual T, Salom JB, Torregrosa G, Miranda FJ, Alborch E (2013) Involvement of prostacyclin and potassium channels in the diabetes-induced hyporeactivity of the rabbit carotid artery to B-type natriuretic peptide. Eur J Pharmacol 701:159–167
Dong DL, Bai YL, Cai BZ (2016) Calcium-activated potassium channels: potential target for cardiovascular diseases. Adv Protein Chem Struct Biol 104:233–261
Ellinsworth DC, Shukla N, Fleming I, Jeremy JY (2014) Interactions between thromboxane A2, thromboxane/prostaglandin (TP) receptors, and endothelium-derived hyperpolarization. Cardiovasc Res 102:9–16
Feng Y, Wang D, Bi H, Zhang H (2016) The role of natriuretic peptides in diabetes and its complications. Biomed Pharmacother 84:1826–1832
Garland CJ, Dora KA (2017) EDH: endothelium-dependent hyperpolarization and microvascular signaling. Acta Physiol (Oxf) 219:152–161
Gokina NI, Bonev AD, Phillips J, Gokin AP, Veilleux K, Oppenheimer K, Goloman G (2015) Impairment of IKCa channels contributes to uteroplacental endothelial dysfunction in rat diabetic pregnancy. Am J Physiol Heart Circ Physiol 309:H592–H604
Gruden G, Landi A, Bruno G (2014) Natriuretic peptides, heart, and adipose tissue: new findings and future developments for diabetes research. Diabetes Care 37:2899–2908
Holcberg G, Kossenjans W, Brewer A, Miodovnik M, Myatt L (1995) The action of two natriuretic peptides (atrial natriuretic peptide and brain natriuretic peptide) in the human placental vasculature. Am J Obstet Gynecol 172:71–77
Huang C, Pollock CA, Chen XM (2015) KCa3.1: a new player in progressive kidney disease. Curr Opin Nephrol Hypertens 24:61–66
Hyman AL, De Witt BJ, Gumusel B, Hao Q, Kadowitz PJ, Lippton HL (2001) l-NAME enhances responses to atrial natriuretic peptide in the pulmonary vascular bed of the cat. J Appl Physiol (1985) 90:2101–2108
Liu Y, Xie A, Singh AK, Ehsan A, Choudhary G, Dudley S, Sellke FW, Feng J (2015) Inactivation of endothelial small/intermediate conductance of calcium-activated potassium channels contributes to coronary arteriolar dysfunction in diabetic patients. J Am Heart Assoc 4:e002062
Malek V, Gaikwad AB (2017) Neprilysin inhibitors: a new hope to halt the diabetic cardiovascular and renal complications? Biomed Pharmacother 90:752–759
Marrachelli VG, Miranda FJ, Centeno JM, Burguete MC, Castelló-Ruiz M, Jover-Mengual T, Pérez AM, Salom JB, Torregrosa G, Alborch E (2010) Mechanisms involved in the relaxant action of testosterone in the renal artery from male normoglycemic and diabetic rabbits. Pharmacol Res 61:149–156
Marrachelli VG, Centeno JM, Miranda I, Castelló-Ruiz M, Burguete MC, Jover-Mengual T, Salom JB, Torregrosa G, Miranda FJ, Alborch E (2012) Diabetes impairs the atrial natriuretic peptide relaxant action mediated by potassium channels and prostacyclin in the rabbit renal artery. Pharmacol Res 66:392–400
Marrachelli VG, Miranda FJ, Centeno JM, Miranda I, Castelló-Ruiz M, Burguete MC, Jover-Mengual T, Salom JB, Torregrosa G, Alborch E (2011) Mechanisms underlying the diabetes-induced hyporeactivity of the rabbit carotid artery to atrial natriuretic peptide. Pharmacol Res 63:190–198
McNeish AJ, Jimenez-Altayo F, Cottrell GS, Garland CJ (2012) Statins and selective inhibition of Rho kinase protect small conductance calcium-activated potassium channel function (KCa2.3) in cerebral arteries. PLoS One 7:e46735
Nakagawa O, Ogawa Y, Itoh H, Suga S, Komatsu Y, Kishimoto I, Nishino K, Yoshimasa T, Nakao K (1995) Rapid transcriptional activation and early mRNA turnover of brain natriuretic peptide in cardiocyte hypertrophy. Evidence for brain natriuretic peptide as an “emergency” cardiac hormone against ventricular overload. J Clin Invest 96:1280–1287
Nishikimi T, Kuwahara K, Nakao K (2011) Current biochemistry, molecular biology, and clinical relevance of natriuretic peptides. J Cardiol 57:131–140
Otsuka K, Tanaka H, Horinouchi T, Koike K, Shigenobu K, Tanaka Y (2002) Functional contribution of voltage-dependent and Ca2+ activated K+ (BKCa) channels to the relaxation of guinea-pig aorta in response to natriuretic peptides. J Smooth Muscle Res 38:117–129
Pandey KN (2008) Emerging roles of natriuretic peptides and their receptors in pathophysiology of hypertension and cardiovascular regulation. J Am Soc Hypertens 2:210–226
Potter LR, Abbey-Hosch S, Dickey DM (2006) Natriuretic peptides, their receptors, and cyclic guanosine monophosphate-dependent signaling functions. Endocr Rev 27:47–72
Ronco C (2010) Cardiorenal syndromes: definition and classification. Contrib Nephrol 164:33–38
Sabrane K, Kruse MN, Gazinski A, Kuhn M (2009) Chronic endothelium-dependent regulation of arterial blood pressure by atrial natriuretic peptide: role of nitric oxide and endothelin-1. Endocrinology 150:2382–2387
Schach C, Resch M, Schmid PM, Riegger GA, Endemann DH (2014) Type 2 diabetes: increased expression and contribution of IKCa channels to vasodilation in small mesenteric arteries of ZDF rats. Am J Physiol Heart Circ Physiol 307:H1093–H1102
Seki N, Nishimura M, Matsumoto T, Fukazawa M, Kenmochi T (2013) Relationship between BNP level and renal function in diabetic nephropathy with microalbuminuria. J Diabetes Complicat 27:92–97
Seki N, Matsumoto T, Fukazawa M (2015) Relationship between the brain natriuretic peptide (BNP) level and remission of diabetic nephropathy with microalbuminuria: a 3-year follow-up study. Horm Metab Res 47:138–144
Sorensen CM, Braunstein TH, Holstein-Rathlou NH, Salomonsson M (2012) Role of vascular potassium channels in the regulation of renal hemodynamics. Am J Physiol Renal Physiol 302:F505–F518
Stitham J, Hwa J (2016) Prostacyclin, atherothrombosis and diabetes mellitus: physiologic and clinical considerations. Curr Mol Med 16:328–342
Taskapan H, Taskapan MC, Orman I, Ulutas O, Yigit A, Ozyalin F, Yologlu S (2013) NGAL and NT-proBNP levels in diabetic patients with macroproteinuria. Ren Fail 35:1273–1277
Theilade S, Hansen TW, Goetze JP, Rossing P (2015) Increased plasma concentrations of midregional proatrial natriuretic peptide is associated with risk of cardiorenal dysfunction in type 1 diabetes. Am J Hypertens 28:772–779
Troncoso Brindeiro CM, Fallet RW, Lane PH, Carmines PK (2008) Potassium channel contributions to afferent arteriolar tone in normal and diabetic rat kidney. Am J Physiol Renal Physiol 295:F171–F178
Troncoso Brindeiro CM, Lane PH, Carmines PK (2012) Tempol prevents altered K+ channel regulation of afferent arteriolar tone in diabetic rat kidney. Hypertension 59:657–664
Woodard GE, Rosado JA (2008) Natriuretic peptides in vascular physiology and pathology. Int Rev Cell Mol Biol 268:59–93
Vallon V, Thomson SC (2012) Renal function in diabetic disease models: the tubular system in the pathophysiology of the diabetic kidney. Annu Rev Physiol 74:351–375
van der Zander K, Houben AJ, Kroon AA, De Mey JG, Smits PA, de Leeuw PW (2002) Nitric oxide and potassium channels are involved in brain natriuretic peptide induced vasodilatation in man. J Hypertens 20:493–499
Vesely DL, Gower WR Jr, Dietz JR, Overton RM, Clark LC, Antwi EK, Farese RV (1999) Elevated atrial natriuretic peptides and early renal failure in type 2 diabetic Goto-Kakizaki rats. Metabolism 48:771–778
Volpe M (2014) Natriuretic peptides and cardio-renal disease. Int J Cardiol 176:630–639
Zhao LM, Zhang W, Wang LP, Li GR, Deng XL (2012) Advanced glycation end products promote proliferation of cardiac fibroblasts by upregulation of KCa3.1 channels. Pflugers Arch 464:613–621
Zhao LM, Su XL, Wang Y, Li GR, Deng XL (2013) KCa3.1 channels mediate the increase of cell migration and proliferation by advanced glycation end products in cultured rat vascular smooth muscle cells. Lab Investig 93:159–167
Zhou HL, Fiscus RR (1989) Brain natriuretic peptide (BNP) causes endothelium-independent relaxation and elevation of cyclic GMP in rat thoracic aorta. Neuropeptides 14:161–169
Acknowledgements
The authors are grateful to Salvador Banacloche for his technical assistance.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical approval
Housing conditions and experimental procedures were performed in strict compliance with the European Union (Directive 2010/63/EU) and Spanish (RD 53/2013) regulations on the use of animals for scientific purposes and approved by the Ethics Committee for Animal Experimentation and Welfare from the University of Valencia (ref. A11295344586921).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Centeno, J.M., Miranda-Gómez, L., López-Morales, M.A. et al. Diabetes modifies the role of prostanoids and potassium channels which regulate the hypereactivity of the rabbit renal artery to BNP. Naunyn-Schmiedeberg's Arch Pharmacol 391, 501–511 (2018). https://doi.org/10.1007/s00210-018-1478-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-018-1478-4