Abstract
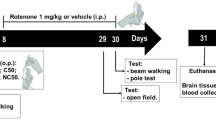
The objective of the present study was to evaluate the protective effect of resveratrol nanoparticles (NRSV) against rotenone-induced neurodegeneration in rats. NRSV were prepared by temperature-controlled antisolvent precipitation method and characterized for its particle size, shape, and dissolution properties. Moreover, NRSV effects compared with the free resveratrol (RSV). Animals were divided into four groups: (I) control, (II) rotenone (2 mg/kg s.c.), (III) RSV (40 mg/kg, p.o.) + rotenone, and (IV) NRSV (40 mg/kg, p.o.) + rotenone. Animals received treatments 30 min before rotenone administration for a period of 35 days. Behavioral quantifications were done using rota rod test and rearing behavior after 24 h of last dose. Animals were euthanized, and mid brains were isolated for the estimation of tricarboxylic acid cycle enzymes, oxidative measures (lipid peroxidation (LPO), glutathione (GSH), and catalase), and complex-I activity. In addition, histopathological studies were also performed. Our results showed that chronic rotenone treatment causes motor deficits, decreased rearing behavior, mitochondrial dysfunction, and oxidative stress. Furthermore, histological analysis demonstrated neuronal degeneration in rotenone-treated rats. An important finding of the present study was NRSV showed comparatively better efficacy than the RSV treatment in attenuating the rotenone-induced Parkinson’s like behavioral alterations, biochemical and histological changes, oxidative stress, and mitochondrial dysfunction in rats.






Similar content being viewed by others
References
Alam M, Schmidt WJ (2002) Rotenone destroys dopaminergic neurons and induces parkinsonian symptoms in rats. Behav Brain Res 136(1):317–324. https://doi.org/10.1016/S0166-4328(02)00180-8
Banji D, Banji OJ, Dasaroju S, Kranthi KC (2013) Curcumin and piperine abrogate lipid and protein oxidation induced by D-galactose in rat brain. Brain Res 1515:1–11. https://doi.org/10.1016/j.brainres.2013.03.023
Beer RF, Seizer TW (1951) A spectrophotometric method for measuring breakdown of hydrogen peroxide by catalase. J Biol Chem 115:130–140
Betarbet R, Sherer TB, MacKenzie G, Garcia-Osuna M, Panov AV, Greenamyre JT (2000) Chronic systemic pesticide exposure reproduces features of Parkinson’s disease. Nat Neurosci 3(12):1301–1306. https://doi.org/10.1038/81834
Bezard E, Yue Z, Kirik D, Spillantini MG (2013) Animal models of Parkinson’s disease limits and relevance to neuroprotection studies. Mov Disord 28(1):61–70. https://doi.org/10.1002/mds.25108
Bueler H (2009) Impaired mitochondrial dynamics and function in the pathogenesis of Parkinson's disease. Exp Neurol 218(2):235–246. https://doi.org/10.1016/j.expneurol.2009.03.006
Bulaj G, Kortemme T, Goldenberg DP (1998) Ionization-reactivity relationships for cysteine thiols in polypeptides. Biochemistry 37:8965–8972
Cai J, Yang J, Jones DP (1998) Mitochondrial control of apoptosis: the role of cytochrome c. Biochim Biophys Acta 1366:139–149
Chen RS, Wu PL, Chiou RYY (2002) Peanut roots as a source of resveratrol. J Agric Food Chem 50(6):1665–1667. https://doi.org/10.1021/jf011134e
Dokoumetzidis A, Macheras P (2006) A century of dissolution research: from Noyes and Whitney to the biopharmaceutics classification system. Int J Pharm 321(1-2):1–11. https://doi.org/10.1016/j.ijpharm.2006.07.011
Ellman GL (1959) Tissue sulfhydryl groups. Arch Biochem Biophys 82(1):70–77. https://doi.org/10.1016/0003-9861(59)90090-6
Hanada S, Fujioka K, Inoue Y, Kanaya F, Manome Y, Yamamoto K (2014) Cell-based in vitro blood–brain barrier model can rapidly evaluate nanoparticles’ brain permeability in association with particle size and surface modification. Int J Mol Sci 15(2):1812–1825. https://doi.org/10.3390/ijms15021812
Hao J, Gao Y, Zhao J, Zhang J, Li Q, Zhao Z, Liu J (2015) Preparation and optimization of resveratrol nanosuspensions by antisolvent precipitation using Box-Behnken design. AAPS PharmSciTech 16:118–128
Hatefi Y, Rieske JS (1967) Preparation and properties of DPNH-coenzyme Q reductase (complex I of the respiratory chain). Methods Enzymol 10:235–239. https://doi.org/10.1016/0076-6879(67)10046-3
Hu LF, Lu M, Tiong CX, Dawe GS, Hu G, Bian JS (2010) Neuroprotective effects of hydrogen sulfide on Parkinson’s disease rat models. Aging Cell 9(2):135–146. https://doi.org/10.1111/j.1474-9726.2009.00543.x
Jin F, Wu Q, Lu YF, Gong QH, Shi JS (2008) Neuroprotective effect of resveratrol on 6-OHDA-induced Parkinson’s disease in rats. Eur J Pharmacol 600(1-3):78–82. https://doi.org/10.1016/j.ejphar.2008.10.005
Kairisalo M, Bonomo A, Hyrskyluoto A, Mudò G, Belluardo N, Korhonen L, Lindholm D (2011) Resveratrol reduces oxidative stress and cell death and increases mitochondrial antioxidants and XIAP in PC6.3-cells. Neurosci Lett 488:263–266
Kesisoglou F, Panmai S, Wu Y (2007) Nanosizing-oral formulation development and biopharmaceutical evaluation. Adv Drug Deliv Rev 59(7):631–644. https://doi.org/10.1016/j.addr.2007.05.003
Khurana N, Gajbhiye A (2013) Ameliorative effect of Sida cordifolia in rotenone induced oxidative stress model of Parkinson’s disease. Neurotoxicology 39:57–64. https://doi.org/10.1016/j.neuro.2013.08.005
Kim S, Ng WK, Dong Y, Das S, Tan RBH (2012) Preparation and physicochemical characterization of trans-resveratrol nanoparticles by temperature-controlled antisolvent precipitation. J Food Eng 108(1):37–42. https://doi.org/10.1016/j.jfoodeng.2011.07.034
King TE, Ohnishi T, Winter DB, Wu JT (1976) Biochemical and EPR probes for structure–function studies of iron sulfur centers of succinate dehydrogenase. Adv Exp Med Biol 74:182–227. https://doi.org/10.1007/978-1-4684-3270-1_15
Kudin AP, Bimpong-Buta NY, Vielhaber S, Elger CE, Kunz WS (2004) Characterization of superoxide-producing sites in isolated brain mitochondria. J Biol Chem 279(6):4127–4135. https://doi.org/10.1074/jbc.M310341200
Lu X, Xu H, Sun B, Zhu Z, Zheng D, Li X (2013) Enhanced neuroprotective effects of resveratrol delivered by nanoparticles on hydrogen peroxide-induced oxidative stress in rat cortical cell culture. Mol Pharm 10(5):2045–2053. https://doi.org/10.1021/mp400056c
Mathieu L, Costa AL, Le Bachelier C, Slama A, Lebre AS, Taylor RW, Bastin J, Djouadi F (2016) Resveratrol attenuates oxidative stress in mitochondrial complex I deficiency: involvement of SIRT3. Free Radic Biol Med 96:190–198. https://doi.org/10.1016/j.freeradbiomed.2016.04.027
Mizuno Y, Yoshino H, Ikebe S, Hattori N, Kobayashi T, Shimoda-Matsubayashi S, Matsumine H, Kondo T (1998) Mitochondrial dysfunction in Parkinson’s disease. Ann Neurol 44:99–109
Mokni M, Elkahoui S, Limam F, Amri M, Aouani E (2007) Effect of resveratrol on antioxidant enzyme activities in the brain of healthy rat. Neurochem Res 32:981–987
Moreadith RW, Fiskum G (1984) Isolation of mitochondria from ascites tumor cells permeabilized with digitonin. Anal Biochem 137(2):360–367. https://doi.org/10.1016/0003-2697(84)90098-8
Mosharrafand M, Nystrom C (1995) The effect of particle size and shape on the surface specific dissolution rate of microsized practically insoluble drugs. Int J Pharm 122(1-2):35–47. https://doi.org/10.1016/0378-5173(95)00033-F
Olanow CW, Agid Y, Mizuno Y et al (2004) Levodopa in the treatment of Parkinson's disease: current controversies. Mov Disord 19(9):997–1005
Queiroz AN, Gomes BA, Moraes WM, Borges RS (2009) A theoretical antioxidant pharmacophore for resveratrol. Eur J Med Chem 44(4):1644–1649. https://doi.org/10.1016/j.ejmech.2008.09.023
Racker E (1950) Spectrophotometric measurement of the enzymatic formation of fumaric and cis-aconitic acids. Biochim Biophys Acta 4(1-3):211–214. https://doi.org/10.1016/0006-3002(50)90026-6
Ruiz-Larrea MB, Leal AM, Liza M, Lacort M, de Groot H (1994) Antioxidant effects of estradiol and 2-hydroxyestradiol on iron induced lipid peroxidation of rat liver microsomes. Steroids 59(6):383–388
Sanchez-Fidalgo S, Cardeno A, Villegas I, Talero E, de la Lastra CA (2010) Dietary supplementation of resveratrol attenuates chronic colonic inflammation in mice. Eur J Pharmacol 633:78–84
Saraiva C, Praça C, Ferreira R, Santos T, Ferreira L, Bernardino L (2016) Nanoparticle-mediated brain drug delivery: overcoming blood–brain barrier to treat neurodegenerative diseases. J Control Release 235:34–47. https://doi.org/10.1016/j.jconrel.2016.05.044
Saravanan KS, Sindhu KM, Mohanakumar KP (2005) Acute intranigral infusion of rotenone in rats causes progressive biochemical lesions in the striatum similar to Parkinson’s disease. Brain Res 1049(2):147–155. https://doi.org/10.1016/j.brainres.2005.04.051
Schapira AH, Copper JM, Clark JB, Jenner P, Marsden CD (1990) Mitochondrial complex-I deficiency in Parkinson’s disease. J Neurochem 54(3):823–827. https://doi.org/10.1111/j.1471-4159.1990.tb02325.x
Shantha KL, Harding DRK (2000) Preparation and in vitro evaluation of poly (Nvinyl-2-pyrrolidone-polyethylene glycol diacrylate)-chitosan interpolymeric pH responsive hydrogels for oral drug delivery. Int J Pharm 207(1-2):65–70. https://doi.org/10.1016/S0378-5173(00)00533-0
Sonmez U, Sonmez A, Erbil G, Tekmen I, Baykara B (2007) Neuroprotective effects of resveratrol against traumatic brain injury in immature rats. Neurosci Lett 420:133–137
Spuch C, Saida O, Navarro C (2012) Advances in the treatment of neurodegenerative disorders employing nanoparticles. Recent Pat Drug Deliv Formul 6(1):2–18
Srere PA (1969) Citrate synthase. In: Lowenstein JM (ed) Methods in enzymology, citric acid cycle. Academic, New York, pp 3–11. https://doi.org/10.1016/0076-6879(69)13005-0
Swarnkar S, Singh S, Sharma S, Mathur R, Patro IK, Nath C (2011) Rotenone induced neurotoxicity in rat brain areas: a histopathological study. Neurosci Lett 501(3):123–127. https://doi.org/10.1016/j.neulet.2011.03.036
Tapias V, Cannon JR, Greenamyre JT (2014) Pomegranate juice exacerbates oxidative stress and nigrostriatal degeneration in Parkinson’s disease. Neurobiol Aging 35(5):1162–1176. https://doi.org/10.1016/j.neurobiolaging.2013.10.077
Uversky VN (2004) Neurotoxicant induced animal models of Parkinson’s disease: understanding the role of rotenone, maneb and paraquat in neurodegeneration. Cell Tissue Res 318(1):225–241. https://doi.org/10.1007/s00441-004-0937-z
Verma R, Nehru B (2009) Effect of centrophenoxine against rotenone induced oxidative stress in an animal model of Parkinson’s disease. Neurochem Int 55(6):369–375. https://doi.org/10.1016/j.neuint.2009.04.001
Wang H, Liu J, Gao G, Wu X, Wang X, Yang H (2016) Protection effect of piperine and piperlonguminine from Piper longum L. alkaloids against rotenone-induced neuronal injury. Brain Res 1639:214–227. https://doi.org/10.1016/j.brainres.2015.07.029
von Wrangel C, Schwabe K, John N, Krauss JK, Alam M (2015) The rotenone-induced rat model of Parkinson’s disease: behavioral and electrophysiological findings. Behav Brain Res 279:52–61. https://doi.org/10.1016/j.bbr.2014.11.002
Zeng W, Zhang W, Lu F, Gao L, Gao G (2017) Resveratrol attenuates MPP+-induced mitochondrial dysfunction and cell apoptosis via AKT/GSK-3β pathway in SN4741 cells. Neurosci Lett 637:50–56. https://doi.org/10.1016/j.neulet.2016.11.054
Zykova TA, Zhu F, Zhai X, Ma WY, Ermakova SP, Lee KW, Bode AM, Dong Z (2008) Resveratrol directly targets COX-2 to inhibit carcinogenesis. Mol Carcinog 47(10):797–805. https://doi.org/10.1002/mc.20437
Acknowledgments
University College of Pharmaceutical Sciences, Kakatiya University, support for the routine reagents and permission to animal holding for this research.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Palle, S., Neerati, P. Improved neuroprotective effect of resveratrol nanoparticles as evinced by abrogation of rotenone-induced behavioral deficits and oxidative and mitochondrial dysfunctions in rat model of Parkinson’s disease. Naunyn-Schmiedeberg's Arch Pharmacol 391, 445–453 (2018). https://doi.org/10.1007/s00210-018-1474-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00210-018-1474-8