Abstract
The continuous increase in the popularity of tattoos and permanent make-up (PMU) has led to substantial changes in their societal perception. Besides a better understanding of pathological conditions associated with the injection of highly diverse substances into subepidermal layers of the skin, their regulation has occupied regulatory bodies around the globe. In that sense, current regulatory progress in the European Union is an exemplary initiative for improving the safety of tattooing. On one hand, the compilation of market surveillance data has provided knowledge on hazardous substances present in tattoo inks. On the other hand, clinical data gathered from patients enabled correlation of adverse reactions with certain substances. Nevertheless, the assessment of risks remains a challenge due to knowledge gaps on the biokinetics of highly complex inks and their degradation products. This review article examines the strategies for regulating substances in tattoo inks and PMU in light of their potential future restriction in the frame of the REACH regulation. Substance categories are discussed in terms of their risk assessment and proposed concentration limits.
Similar content being viewed by others
Introduction
Surveys conducted in recent years have shown a continuous increase in the prevalence of tattoos and permanent make-up (PMU). The estimated tattooed population is about 12% in the EU, reaching up to 25% for the age group of 18–20 years (BfR 2018; JRC 2016b; Renzoni et al. 2018). In the US, 33% of the general population bear at least one tattoo, reaching up to 38% for ages 18–29 (Bäumler 2016; Breuner and Levine 2017; Tighe et al. 2017). Although tattoos of different kinds date back to the earliest stages of tribal communities and rituals, it is only in recent times that the scientific community and regulatory authorities were inclined to recognize their solid place in modern society (Kasten 2007). All together, these developments have led to institutional attention and the attempt to establish harmonized and effectual regulations for tattoos and PMU materials.
Tattoo inks are manufactured by mixing pigments with auxiliary compounds that control viscosity, drying properties, homogeneity in terms of particle sedimentation, and shelf life of the ink. The common components of tattoo inks are summarized in Scheme 1. Pigments are mostly manufactured for large-scale applications in the automotive, construction, or cosmetics industries (Laux et al. 2016) rather than specifically for tattooing. Therefore, the safety for their application in tattoo inks is not evaluated in particular. The injection of pigments into the human dermis as an ingredient of tattoo inks has actually neither considered nor even opposed by pigment manufacturers. A comprehensive evaluation of the safety of each product represents a great challenge for the rather minor sector of tattoo ink producers who are specialists for the formulation. Altogether, this underlines the urgent necessity for the regulation of tattoo ink constituents. The extent of pathological conditions directly related to the exposure to tattoo inks is generally difficult to estimate as only approximately 50% of those individuals that develop complications request medical advice (Renzoni et al. 2018). An additional reason for an inaccurate estimation of the prevalence of pathological conditions originates from the difficulty to connect certain systemic effects to the toxicity of tattoo ink ingredients or their degradation products. Although local reactions can undoubtedly be attributed to the site of the tattoo, the respective causative substances remain mostly undiscovered.
Despite the presence of well-known carcinogens, to date, no local or systemic carcinogenicity could be directly attributed to the exposure to tattoo ink ingredients (EC 2019; Kluger and Koljonen 2012; Sabbioni and Hauri 2016). Although various malignancies and benign tumors were localized in the tattooed skin areas, it remains unclear whether these were triggered by the induced trauma, the inflammatory reaction, the chemical composition of the tattoo ink, or coincidentally (Kluger 2017; Kluger and Koljonen 2012; Munshi et al. 2011). A causal proof or exclusion of their cancer-promoting potency in the skin or internal organs of tattooed individuals would require well-defined, long-term epidemiological studies though. The vast majority of complications are related to local skin reactions. About 68% of individuals report skin problems immediately or a few weeks after receiving a tattoo (Klügl et al. 2010). In most cases, it is challenging to differentiate between an allergic or corrosive/tissue damaging reaction. Among all tattoo-related complications seen in the clinics, Serup et al. attributed 37% to being of allergic nature (Serup et al. 2016). The purpose of this review is to discuss the state of knowledge available on the biodistribution of tattoo inks and to present the development of tattoo and PMU regulation that has been achieved in the EU in the meantime. Hereby, the main aspects under the REACH (Regulation, Evaluation, Authorization and restriction of CHemicals) regulation will be discussed from a critical perspective, and alternative measures for ensuring the safety of consumers will be presented.
Biodistribution of tattoo inks: in vivo and ex vivo evidence
With regard to the unique exposure scenario of tattooing, the administration of colorants into the human dermis, and the biodistribution of nano- and micro-meter-sized pigment particles have rarely been addressed. Here, most significant in vivo studies, ex vivo skin analyses, as well as the latest evidence obtained from tattooed individuals will be discussed. Tattoo inks are deposited in the dermis, while the depth and amount of pigment deposition varies depending on the density of the ink and the skills of the artist (Engel et al. 2008). Engel et al. estimated the amount of the deposited ink in human and pig skin in the range of 0.4–14.36 mg/cm2 (Arbache et al. 2019; Laux et al. 2016; Prior 2015). Once arrived in the dermis, the pigments and the soluble auxiliary components start undergoing diverse processes. The cellular internalization of tattoo pigments and the role of macrophages were reported in an early study of tattooed rabbits (Mann and Klingmüller 1981). A recently developed mouse model revealed depletion of tissue-resident macrophages upon diphtheria toxin (DT) injection. Using of this mouse model, the life cycle of a tattoo pigment could be investigated (Baranska et al. 2018). In contrast to naïve dendritic cells (DCs), which migrate to draining lymph nodes, monocyte-derived DCs and tissue-resident macrophages remain in the tissue to contribute to the local immune response and tissue homeostasis, respectively (Haniffa et al. 2009, 2012; Tamoutounour et al. 2013). It could be demonstrated that tattoo pigments inserted into the mouse tail remained primarily within the dermal resident macrophages upon endocytosis. Interestingly, in a DT-treated dermis lacking macrophages, the free pigments became readily internalized by the newly generated macrophages originating from circulating monocytes, without major changes in the appearance of the tattoo. Hence, the pigment “capture—release—recapture model” was proposed to explain the persistence of tattoo pigments within the dermis. Based on microscopic investigations only, others suggested that, rather, the fibroblasts and the connective tissue act as main reservoirs for tattoo pigments residing in the dermis of tattooed human skin biopsies (Ferguson et al. 1997) and mice (Fujita et al. 1988).
Due to direct contacts with blood and lymph fluids during the traumatizing procedure, tattoo inks injected into the human dermis must be considered as 100% systemically bioavailable. Especially soluble ingredients of tattoo inks are supposed to be subjected to metabolic processes—although little is known about their metabolites and toxicokinetics. A rapid systemic distribution of soluble tattoo ink components can be assumed. Unlike the insoluble pigments, for soluble auxiliary ingredients, existing toxicologically derived no observed adverse effect levels (NOAELs) can be readily extrapolated from studies that used the dermal application route. Subsequently, the so-called margin of safety (MOS), that is, the ratio between the NOAEL and the estimated exposure level, can be calculated (EDQM 2017).
Despite their persistence in the area of injection, insoluble pigments can be readily found in regional lymph nodes. Pigmentation of the inguinal and axillary lymph nodes as well as the immune response upon tattooing could be demonstrated in SKH-1 mice as a surrogate model for human tattooing (Gopee et al. 2005). The initiated inflammatory process and the accompanying elevated interleukin (IL)-1β and IL-10 levels in the tattooed skin and the regional lymph nodes recovered within 14 days. Since control mice, tattooed with glycerol only, had a similar immune response, the inflammation process induced can be—at least partly—attributed to the injury by the needle and not necessarily to the ingredients of the ink only. The same mouse model was used to evaluate the transport and decomposition of Pigment Red 22 (that is, 3-hydroxy-4-[(2-methyl-5-nitrophenyl)azo]-N-phenylnaphthalene-2-carboxamide) (Engel et al. 2010). 42 days after tattooing, 32% of the pigment had been cleared from the site of injection. Interestingly, this value was almost doubled after exposure of the mice to simulated solar radiation, likely indicating degradation of the pigment and rapid clearance of its metabolites. The relevance of these findings for humans must be interpreted with caution due to the absence of a sub-cutaneous fat layer and the thin skin of mice, where pigments might be more prone to photo-degradation. In that sense, porcine skin was found to act as a more appropriate model for simulating human exposure. This is not only due the thickness of the skin but also to its wound healing properties (Sullivan et al. 2001). In particular, the mini-pig model was established as a non-rodent regulatory tool for predicting human toxicity (Bode et al. 2010).
Some kind of alarming were the results achieved with human skin biopsies, where a clearance rate of 87–99% has been estimated immediately after tattooing (Lehner et al. 2011). However, this value is based on a rather rough estimation of the pigment amount applied initially. Yet, the lymphatic system seems to play a major role in the transport of the applied pigments and its impurities. Black ink could be extracted and analyzed from skin and regional lymph nodes (Lehner et al. 2014). Possibly adsorbed to carbon black, polycyclic aromatic hydrocarbons (PAHs) could be simultaneously detected in concentrations reaching 0.6 μg/cm2 in the skin samples and 11.8 μg/g in the lymph nodes, respectively. Potential synergistic effects on cancer development due to the presence of chemical carcinogens and UV irradiation were studied in mice tattooed with a black ink containing various PAHs including the model carcinogen benzo[a]pyrene (Lerche et al. 2015). Interestingly, skin cancer development was delayed by 50 days in the group of tattooed mice when compared to the control group being irradiated only. In addition, tattooed mice, not exposed to UV radiation, did not develop skin tumors. The authors explained these finding tentatively by the ability of the black ink to absorb UV radiation and hence to reduce the backscattering of the incident light. A similarly designed study investigated mice tattooed with a commercial red ink (Lerche et al. 2017). Here, no inhibition of tumor growth was observed. On the contrary, a group of mice that was tattooed with a red ink contaminated with 2‐anisidine, a primary aromatic amine (PAA), and subsequently exposed to UV light, showed an enhanced tumor growth. Yet, the differences to the control group of mice being irradiated but not tattooed were rather minor. Moreover, for the sake of such comparative studies, analytical characterization and quantification of the pigments and other relevant ingredients and impurities are mandatory for drawing valid conclusions regarding the carcinogenic potential of tattoo inks and the accompanying effects of UV irradiation.
Any correlation between an observed adverse effect and the ink applied requires first and foremost the determination of the chemical identity of the pigments and auxiliary compounds. With the aid of synchrotron X-ray fluorescence (XRF) and advanced mass spectrometry techniques, the intravital distribution of pigments and associated elements was confirmed by their chemical identification in skin and lymph nodes isolated from human corpses (Schreiver et al. 2017). Nonetheless, recent findings suggest that the origin of the deposited metal elements is not only the ink itself, but also attrited particles from tattoo needles. Nano- and micro-meter-sized nickel (Ni) and chromium (Cr) particles were found to be released from the needles and deposited in the skin, especially upon usage of ink containing abrasive titanium dioxide (TiO2). Moreover, translocation of these elements to the local lymph nodes has been revealed (Schreiver et al. 2019).
In a study which attempted to shed light on the systemic distribution of tattoo pigments, skin, lymph nodes, liver, spleen, kidney, and lung of tattooed mice have been analyzed (Sepehri et al. 2017). Mice tattooed with black and red inks were sacrificed after 1 year. In addition to skin and lymph nodes, pigments could be microscopically detected in Kupffer cells of the liver, hence suggesting their distribution via the bloodstream. In this study, no pigments could be localized in other organs though. However, there is further evidence that intradermal and subcutaneous injection of particulate matter may lead to deposition in organs such as liver, kidney, spleen, and hepatic lymph nodes (Gopee et al. 2007; Tang et al. 2009). Tattoo pigments are not eliminated from the site of injection via lymph and blood only. Until regeneration of the skin barrier has ended within about 1 month after tattooing, pigment particles are also eradicated by transepidermal elimination (Shah et al. 2018).
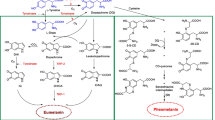
In terms of potential risk to human health, impurities of tattoo inks might be most relevant. These may comprise genotoxins such as certain PAAs, PAHs, nitrosamines, and formaldehyde. Furthermore, heavy metals such as nickel, chromium, and lead have been reported (Forte et al. 2009; Regensburger et al. 2010). Preservatives, a substance group with specific functional properties, may pose human health risks, as well. Adverse effects appearing directly after tattooing or years later have been monitored in dermatological clinics and are comprehensively summarized by Kluger (2019) and Serup et al. (2016). In summary, acute contact dermatitis, inflammatory reactions, and infectious complications account for common acute effects. And yet instant or delayed allergic reactions remain to be the most predominant adverse effect (Laux et al. 2016). Individual cases of patients who developed systemic anaphylaxis shortly after receiving a tattoo have been also reported (Jungmann et al. 2016). The analytical assessment of the applied ink revealed the presence of formaldehyde, parabens, isothiazolinones, and metals such as nickel, cobalt, manganese, cadmium, and antimony. Red inks were often reported for inducing an allergic reaction, although the identification of the specific allergen or the hapten is not necessarily straightforward (Serup et al. 2016; van der Bent et al. 2019; Wenzel et al. 2013). In fact, patients who developed an allergic reaction to a red tattoo did not react to the respective pigment in a patch test (Gaudron et al. 2015; Greve et al. 2003). This might be due to the necessity of the presence of other compounds or metabolites of the original pigment to promote cross activation. Also, the poor penetration of the test substance into the skin may lead to a false-negative readout (Steinbrecher et al. 2004). Recently, the analysis of skin biopsies of patients with allergic reactions against tattoos allowed the identification of organic pigments as well as metal ions with most frequent abundance (Serup et al. 2019). Among the organic pigments, azo class pigments were found in most of the biopsies analyzed. In particular, Pigment Red 22 (C.I number 12315) was found in 35% of the biopsies. The majority of the analyzed samples contained elevated levels of Fe and Cu, but also of Cr, Ti, Mn, Ni, and Cd. Hence, there is evidence of photo-degradation of pigments in human skin and cross reactivity between emerging metabolites and their parent compounds.
Regulation of tattoo ink ingredients: REACH restriction vs. product-specific regulations
In EU countries which do not have their own specific law, currently the regulation of tattoo inks and PMU falls under the General Product Safety Directive (GPSD) (Directive 2001/95/EC). Other aspects such as labeling requirements and registration of chemicals are realized via CLP (Classification, Labeling, and Packaging) (EC No 1272/2008) and REACH (EC No 1907/2006) regulation, respectively (Scheme 2). Due to its non-specific nature, this regulatory framework currently fails to secure the safety of tattoo inks and their impurities. The resolutions developed by the Council of Europe (CoE ResAP(2003)2 and CoE ResAP(2008)1) actually set the basis for the existing national legislation of tattoos and PMU in ten European countries (EC 2003; 2008). The corresponding national legislations in Belgium, France, Germany, Norway, The Netherlands, Slovenia, Spain, Sweden, Switzerland, and Lichtenstein were built with rather minor deviations from the resolutions in terms of restricted colorants, use of preservatives, and other auxiliary substances (JRC 2016b). Nevertheless, given the mobility of products in the EU and in light of a considerable number of RAPEX (Rapid Alert System for dangerous non-food products) announcements, the necessity for an obligatory regulation on the European level becomes obvious. Based on the evidence collected from a large-scale survey, the German Federal Institute for Risk Assessment (BfR) and the Danish Agency for Environmental Protection provided recommendations on the safety of tattoo inks (BfR 2012; EPA 2014). These recommendations contain strategies for safety assessment, limitations for certain classes of substances, as well as labeling and sterility requirements. It is proposed to follow an approach similar to the safety assessment developed by the Scientific Committee on Consumer Safety (SCCS) in the case of cosmetic products (SCCS 2012).
The report of the Joint Research Center (JRC) of the European Commission (EC), compiled by expert stakeholders from research and risk assessment, intended to set a legislative framework for assuring consumer safety. The reports of the four work packages represent the results of collaborative efforts of member states to monitor trends in tattooing, their prevalence, as well as adverse effects linked to their application and removal (JRC 2015a, b, 2016a, b). The main finding of the report was the presence of tattoo inks on the European market which do not comply with the limits set by the recommendations of the Council of Europe [ResAP(2003)2 or ResAP(2008)1]. Moreover, both the absence of suitable analytical methods and the insufficiency of clinical data on systemic or local complications were underlined. Interestingly, along with the reported adverse health reactions, bacterial infections at a rate of 5% were traced back to non-sterile inks or tattoo practice. Different from cosmetic products, tattoo inks are injected directly into the dermis and thus come into direct contact with immune cells, blood, and lymphatic fluid. For that reason, data obtained by the conventional dermal toxicity assays are unlikely to fully predict the toxicity of these substances when injected into human skin.
Referring to the evidence provided by JCR, the European Commission (EC) requested the European Chemical Agency (ECHA) to prepare an annex XV restriction dossier under REACH (Scheme 3). The future restriction of substances in tattoo inks and PMU is presented in the submitted dossier of ECHA, and the subsequent evaluation by the Committees for Risk Assessment (RAC) and Socio-Economic Analysis (SEAC) (ECHA 2019). Here, it is proposed to regulate tattoos and PMU with cross reference to already established legislations. The obvious advantage of such a proposal is the implementation of restriction strategies for readily established substance categories, by the so-called active link strategy. In that way, a harmonized classification of a certain substance would directly apply to tattoo ink ingredients and, by prohibiting the use of the substance, guarantee the absence of a specific chemical hazard in the ink. Yet, whether the safety of a final product can be guaranteed under such guidelines remains unclear. A noticeable obstacle is represented by harmful chemicals which lack harmonized classifications or distinct bans. These may still be used in tattoo inks. On the contrary, substances used for decades in tattoos may be banned due to their classification on substance level or in other product categories.
Without expanding in detail on the scope of the ECHA dossier, the over 4000 restricted substances can be categorized as depicted in Scheme 3. A major number of substances are regulated through the Cosmetic Product Regulation (CPR) (EC) No. 1223/2009. Under the assumption that substances prohibited in cosmetic products are at least as hazardous when injected into the skin, they shall be prohibited in tattoos and PMU, as well. Annex II of CPR (EC) 1223/2009 includes the list of banned substances, while Annex IV sets conditions and concentration limits for colorants allowed in cosmetic products. The controversy of such an assumption lies in the fact that the introduction of substances into the human dermis should actually lead to more strict requirements, given the much higher risks entailed. Furthermore, the ban of some substances in cosmetic products is justified not necessarily based on known toxic effects but rather due to the lack of interest of manufacturers to provide safety data for certain areas of application, as, e.g., for the use of pigment blue 15 (copper phthalocyanine) and pigment green 7 (phthalocyanine green) in hair dyes.
Substances with harmonized entries in Annex VI to Regulation (EC) 1272/2008 are restricted according to the hazard classes, as shown in Table 1. It is important to highlight that substances present in gaseous state at standard temperature and pressure with the mentioned above hazard classification were derogated from the scope of this restriction, with the exception of formaldehyde. However, beside formaldehyde, further hazardous gases such as ammonia or residual monomers of polymers used as auxiliary substances may occur in tattoo inks. Such monomers include compounds like buta-1,3-diene or ethylene oxide. Substances classified as hazardous by inhalation only were left outside the scope of the restriction, as toxicity via other routes of exposure can be ruled out in that case. Conversely, ocular toxicity was considered due to the possible exposure towards tattoo ink and PMU application surrounding the eyelids. Further groups of substances are adopted from the existing resolutions of the Council of Europe. These include PAHs with a harmonized classification as carcinogenic or mutagenic, PAAs of concern, and azo pigments with relevant classifications as CMR (carcinogenic, mutagenic, or toxic for reproduction), and/or skin sensitizers. This further encompasses pigments able to decompose to one of the PAAs of concern, toxic metal impurities, or methanol for its eye damaging potential. Table 2 provides an overview of the substance categories with the corresponding concentration limits according to their currently proposed restriction under REACH in comparison to ResAP(2008)1. In this scope, no specific reference is given with regard to risks associated with photosensitivity, systemic toxicity, or exposure towards nanomaterials.
Although restricted substances may have a threshold under the CLP regulation, their total ban in tattoo inks is justified by the absence of supporting experimental data for their intradermal application. This is of particular relevance for skin sensitizers. Under the assumption that substances administered intradermally have a stronger sensitization effect when compared to conventional exposure routes, no DN(M)EL [Derived No (Minimal) Effect Level] can be derived. Recent literature and the RAC opinion underline that the simultaneous presence of irritants and the damage induced to the skin during tattooing may enhance the sensitization potential of certain substances (Frankild et al. 2000; McFadden and Basketter 2000; RAC 2018; Schwitulla et al. 2014).
CLP Regulation (EC) No 1272/2008 sets specific concentration limits for substances present in mixtures with harmonized classifications as carcinogens of categories 1A, 1B (≤ 0.1%), and 2 (≤ 1.0%), and a few exceptions with lower limits. However, these concentration limits are based solely on the hazard evaluation of the respective substances. No risk assessment or exposure assessment specifically for intradermal injection were made. Hence, independently of the unique application scenario of tattoo inks, the banning of CM classified substances was set based on the practical approach to reduce risks and to prevent their intentional use (RAC 2018). This approach may also be referred to as the qualitative approach which sets the concentration limits based on toxicity data only. Further groups of substances such as PAAs, azo pigments, substances toxic for reproduction, methanol, and metallic impurities are suggested to be regulated according to the semi-quantitative approach based on DN(M)EL value derivation. Here, the exposure scenario considered is based on 300 cm2 of tattooed skin, 14.36 mg ink per cm2 human skin, 25% pigments in the ink, and a body weight of 60 kg (Annex XV Restriction Report, Version 1.1, 2017).
The enforceability of the restriction relies first and foremost on the achievable concentration limits with available analytical methods and reference materials (Niederer et al. 2018). This is also the reason for replacing “shall not contain” by specific concentration limits, aiding the enforceability of the restriction. A compendium of analytical methods in compliance with REACH Annex XVII restrictions is recommended by ECHA’s Forum for Exchange of Information on Enforcement (ECHA 2016). It encompasses more than 100 methods from European or national standardization bodies, official methods published in the legal text of REACH, and internal methods of recognized European reference laboratories. However, to this date, not for all substances restricted under REACH validated (harmonized) analytical methods do exist. This refers particularly to tattoo ink matrices. However, harmonized methods are not always advantageous in comparison to in-house validated methods due to the efforts involved in their establishment. The necessity of a harmonized validation may arise where substance release is regulated, e.g., in the case of PAH extraction from carbon black. Here, sample preparation may vastly determine the level of substance release. The main challenge in analytical method development remains the very low pigment solubility. Since their reliable quantification is not yet possible, specific concentration limits are not appropriate for most pigments. Furthermore, the absence of analytical standards of various pigments impedes their quantification according to the proposed limits. To implement chemical requirements, it remains crucial to achieve further analytical method development and validation.
The proposed declaration requirements cover all substances classified for human health according to Annex I of Regulation 1272/2008 as well as all substances within the scope of the REACH regulation. However, non-classified substances are not required to be stated on the ink bottle as the declaration of self-classified substances according to CLP may lead to different labeling for the same substance (ECHA 2018). Here, pigments without any notifications are excluded from the declaration onus. Nonetheless, the declaration of all ingredients is required according to current law in Germany, and would be beneficial to maintain in terms of transparency, analytical detection, and medical treatment in cases of adverse reactions (TätoV 2008). Furthermore, potential tattoo removal measures are facilitated by knowledge of the respective tattoo pigments. Hence, information on substances lacking classification and being outside the scope of the restriction should still be provided.
The selection of 19 banned pigments was adopted from the CPR, which refers to compounds banned in hair colors. Nonetheless, no sufficient data exist to derive a toxicological hazard or risk for these colorants, since the ban in hair colors is a direct consequence of the failure of the industry to submit an appropriate dossier for toxicological evaluation. This accounts in particular for the widely used Pigment Blue 15:3 and Pigment Green 7. Substitutes for these pigments might be even more harmful but are outside the scope, since they were, so far, never subject to any restriction or regulation. Pigments modified by bromination or chlorination, whose adverse effects are unknown, have also found their way into the market. This phenomenon is well demonstrated based on the findings of Hauri et al. (2014) who found the partially brominated Pigment Green 36 (C.I. 74265), with an abundance of 3.5% among all pigments analyzed. The partially brominated Pigment Green 36 is now used as a substitute for Pigment Green 7 (Hauri 2014; Hauri et al. 2009), and it did not appear in the market surveillance performed back in 2009. In addition, while ResAP(2008)1 defines that tattoo inks shall not contain or release PAAs, the potential PAA release is not addressed under REACH. A general consideration of azo pigments (which may release PAAs) would restrict pigments and PAAs that are currently left outside the scope of the restriction.
Sterility of tattoo inks and use of biocides
Complications related to tattoos can be caused not only by hazardous substances but also by their wrong application resulting in pigment overload or excessive needle traumata (JRC 2016a; Sepehri et al. 2016; Serup et al. 2016). The regulation of tattoo ink ingredients as chemicals with no direct consideration of hygienic aspects based on existing clinical evidence is insufficient to provide comprehensive safety of the consumers. The newly developed European standard for safe and hygienic practice is an evidence-based document which provides guidelines for protecting the consumers and tattoo artists from infections (DIN EN 2017). This norm is foreseen to be officially adopted and published by the European Committee for Standardization (CEN). Although legally non-binding, it covers vital aspects of tattooing practice and communication with health authorities.
Resolution ResAP(2008)1 contains a detailed description of sterility requirements. Manufactured and supplied in sterile containers, tattoo inks may contain biocidal products for preservation after opening, but not for the compensation of insufficient microbiological quality. Currently, tattoo inks must also comply with the Biocidal Product Regulation (BPR) No. 528/2012. It is crucial to underline that no biocidal product listed in the BPR was authorized for its use in tattoo inks. Only preservatives (active substances) listed in Annex I and under the category of product type 6 (preservatives for products during storage, “in-can”) of the BPR may be used in tattoo inks (Table 3). As a consequence, substances with harmonized classification as CMR, skin sensitizers, irritants or corrosives, and eye corrosives or irritants will be excluded from that list. Conversely, preservatives with no harmonized classification are still allowed to be added to tattoo inks, although the absence of classification does not necessarily indicate their safety but rather an absence of data. On the contrary, preservatives with solid toxicological profiles and concentration limits, approved for cosmetic products, will be entirely banned. The classification as skin corrosive indicates an irreversible damage to the skin. However, if a substance is classified as corrosive only due to extreme pH values (≤ 2 or ≥ 11.5), its ban in tattoo inks above a concentration limit of 0.01% is not always justified. Instead, the specific concentration limits for corrosive/irritant substances according to CLP should be considered. This concerns in particular the remaining biocidal substances which are allowed to be used in tattoo inks. The classification as skin irritant refers to the elicitation of reversible erythema, edema, or inflammation. Also for this classification, generic or specific concentration limits have been established within CLP. These should be considered. Some examples of such substances used in cosmetic products are listed in Table 4. The most common animal-based models to evaluate the sensitization potential of a substance are the guinea pig maximization test (GPMT), the mouse local lymph-node assay (LLNA), and the Buehler assay. Skin irritation/corrosion and skin sensitization potential of intradermally applied chemicals are evaluated, among others, as described in the OECD guideline No. 406 and ISO 10993–10 for GPMT. Different from the topical application of the substance, its intradermal application allows a more realistic approximation of the real tattooing scenario. The concentration limits set might, therefore, represent a realistic exposure scenario also in the case of tattoo inks.
The exact limits of harmonized skin irritants and corrosive substances have led to critical comments during the public consultation of the restriction dossier (ECHA 2018). The strict limit for all these substances is especially questionable in view of the concentrations given within Annex IV of REACH for certain substances, e.g., propionic acid. This compound is considered being a safe preservative. Propionic acid is used as an authorized food additive, as a preservative in cosmetics or in hair care products. Although its skin/eye corrosive and irritant properties appear at concentrations way above those used for preservation, its utilization shall be forbidden in tattoo inks according to the proposed REACH restriction.
Requirements for a comprehensive toxicological evaluation
Rather than expanding the restriction of tattoo inks to excessive lists of chemicals by creating cross links to existing regulations and intensifying analysis of a large number of chemicals, comprehensive safety can be guaranteed by the consideration of toxicological substance profiles in combination with their exposure. Currently, some 4130 substances are subject of the REACH regulation of tattoo inks. This list can certainly be shortened to a collection of several dozens, when considering the non-substitutable pigments, the effective and safe preservatives, and a selection of auxiliary compounds. Niederer et al. identified 18 tattoo and 10 PMU inks that have been most frequently used on the Swiss market between the years 2009 and 2017 (Niederer et al. 2018). The European Directorate for the Quality of Medicines (EDQM) has published an extensive list of pigments found on the European market between the years 2003 and 2013 (EDQM 2017). This list comprises 49 pigments found in the frame of market surveillance measures in the Netherlands, Germany, Denmark, Norway, and Switzerland. With regard to the use of preservatives, the Norwegian positive list for preservatives with low sensitization potential comprises 21 substances. These examples indicate that most of the substances considered by the REACH restriction are not being used in tattoo inks. And yet more alerting is the fact that substances used in tattoo inks or newly developed products entering the market will remain outside the scope of any regulation based on negative lists.
The support of regulation of tattoo inks via selective positive lists rather than unselective negative lists has previously been suggested by governmental institutions, industry, and individuals in the phase of public consultation (ECHA 2018). The requirements for a comprehensive evaluation of tattoo ink safety were likewise described by statements of the German Federal Institute for Risk Assessment and the EDQM (BfR 2012; EDQM 2017). Nonetheless, a major obstacle for accomplishing a comprehensive toxicological evaluation remains the choice of the experimental models for biokinetics, systemic toxicity, or organ distribution. At present, not all health risks can be addressed via available in vitro or in silico methods. The adopted Directive 2010/63/EU of the European parliament and of the Council for Protection of Animals used for Scientific Purposes supports the generation of alternatives to animal testing. A full ban of animal testing for finished cosmetic products and cosmetic ingredients entered into force in 2013. Hence, also the performance of animal experiments of products based on voluntary exposure remains questionable. Meanwhile, further development of sensitive analytical techniques and the collection of human biomonitoring data represent important elements to address the main open questions regarding absorption, distribution, metabolism, and excretion of tattoo ink ingredients and their degradation products.
Conclusions
The efforts of regulatory authorities to ensure a safe practice of tattooing have led to enormous progress in the understanding of the life cycle of substances injected underneath the epidermal layer of the skin. Along with that knowledge, many questions remain unresolved, in particular regarding degradation products and their systemic bioavailability. It seems obvious that only a stand-alone legislation of tattoo inks may encompass the diverse aspects of tattooing such as their ingredients, sterility, labeling requirements, and appropriate training of tattoo artists. Such a restriction should be based on thoroughly evaluated target substances. The scope of the dossier prepared by ECHA represents a common European initiative and certainly provides an important step towards a legislative framework for the restriction of non-intentionally added compounds, i.e., impurities. Its implementation and effectiveness should be carefully monitored and further developed.
References
Arbache S, Mattos EDC, Diniz MF et al (2019) How much medication is delivered in a novel drug delivery technique that uses a tattoo machine? Int J Dermatol 58(6):750–755. https://doi.org/10.1111/ijd.14408
Baranska A, Shawket A, Jouve M et al (2018) Unveiling skin macrophage dynamics explains both tattoo persistence and strenuous removal. J Exp Med 215(4):1115–1133. https://doi.org/10.1084/jem.20171608
Bäumler W (2016) Tattoos and their potential health consequences. Dtsch Arztebl Int 113(40):663–664. https://doi.org/10.3238/arztebl.2016.0663
BfR (2012) Requirements for tattoo inks. BfR opinion No 013/2013. https://mobil.bfr.bund.de/cm/349/requirements-for-tattoo-inks.pdf
BfR (2018) BfR-Verbrauchermonitor 2018, Spezial Tattoos. https://www.bfr.bund.de/cm/350/bfr-verbrauchermonitor-2018-spezial-tattoos.pdf
Bode G, Clausing P, Gervais F et al (2010) The utility of the minipig as an animal model in regulatory toxicology. J Pharmacol Toxicol Methods 62(3):196–220. https://doi.org/10.1016/j.vascn.2010.05.009
Breuner CC, Levine DA (2017) Adolescent and young adult tattooing, piercing, and scarification. Pediatrics. https://doi.org/10.1542/peds.2017-1962
DIN EN (2017) 17169:2017-10 - draft, tattooing - safe and hygienic practice. German and English version prEN 17169:2017. https://doi.org/10.31030/2690826
EC (2003) Resolution ResAP(2003)2 on tattoos and permanent make-up. https://search.coe.int/cm/Pages/result_details.aspx?ObjectId=09000016805df8e5. Accessed 3 Feb 2020
EC (2008) Resolution ResAP(2008)1 on requirements and criteria for the safety of tattoos and permanent make-up. https://rm.coe.int/16805d3dc4. Accessed 3 Feb 2020
EC (2019) Rapid alert system for dangerous non-food products. https://ec.europa.eu/consumers/consumers_safety/safety_products/rapex/alerts/?event=main.search&lng=en#searchResults. Accessed July 2019
ECHA (2016) Compendium of analytical methods Recommended by the Forum to check compliance with Reach annex xvii restrictions. https://doi.org/10.2823/399943, https://echa.europa.eu/documents/10162/13577/compendium_of_analytical_methods_en.pdf
ECHA (2018) Response to comments document (RCOM) on the Annex XV dossier proposing restriction on Substances used in tattoo inks and permanent make-up. Public consultation on Annex XV report started on 20/12/2017. https://echa.europa.eu/de/registry-of-restriction-intentions/-/dislist/details/0b0236e180dff62a
ECHA (2019) Substances in tattoo inks and permanent make up. European Chemicals Agency, Helsinki
EDQM (2017) Safer tattooing—overview of current knowledge and challenges of toxicological assessment. European Directorate for the Quality of Medicines and HealthCare of the Council of Europe, Strasbourg
Engel E, Santarelli F, Vasold R et al (2008) Modern tattoos cause high concentrations of hazardous pigments in skin. Contact Dermat 58(4):228–233. https://doi.org/10.1111/j.1600-0536.2007.01301.x
Engel E, Vasold R, Santarelli F et al (2010) Tattooing of skin results in transportation and light-induced decomposition of tattoo pigments—a first quantification in vivo using a mouse model. Exp Dermatol 19(1):54–60. https://doi.org/10.1111/j.1600-0625.2009.00925.x
EPA (2014) Recommendation from the Danish Environmental Protection Agency on the safety of tattoo ink. The Danish Environmental Protection Agency, Copenhagen
Ferguson JE, Andrew SM, Jones CJP, August PJ (1997) The Q-switched neodymium: YAG laser and tattoos: a microscopic analysis of laser-tattoo interactions. Br J Dermatol 137(3):405–410. https://doi.org/10.1046/j.1365-2133.1997.18581951.x
Forte G, Petrucci F, Cristaudo A, Bocca B (2009) Market survey on toxic metals contained in tattoo inks. Sci Total Environ 407(23):5997–6002. https://doi.org/10.1016/j.scitotenv.2009.08.034
Frankild S, Volund A, Wahlberg JE, Andersen KE (2000) Comparison of the sensitivities of the Buehler test and the guinea pig maximization test for predictive testing of contact allergy. Acta Dermato-Venereol 80(4):256–262. https://doi.org/10.1080/000155500750012126
Fujita H, Nishii Y, Yamashita K, Kawamata S, Yoshikawa K (1988) The uptake and long-term storage of india ink particles and latex beads by fibroblasts in the dermis and subcutis of mice, with special regard to the non-inflammatory defense reaction by fibroblasts. Arch Histol Cytol 51(3):285–294. https://doi.org/10.1679/aohc.51.285
Gaudron S, Ferrier-Le Bouedec MC, Franck F, D'Incan M (2015) Azo pigments and quinacridones induce delayed hypersensitivity in red tattoos. Contact Dermat 72(2):97–105. https://doi.org/10.1111/cod.12317
Gopee NV, Cui Y, Olson G et al (2005) Response of mouse skin to tattooing: use of SKH-1 mice as a surrogate model for human tattooing. Toxicol Appl Pharmacol 209(2):145–158. https://doi.org/10.1016/j.taap.2005.04.003
Gopee NV, Roberts DW, Webb P et al (2007) Migration of intradermally injected quantum dots to sentinel organs in mice. Toxicol Sci 98(1):249–257. https://doi.org/10.1093/toxsci/kfm074
Greve B, Chytry R, Raulin C (2003) Contact dermatitis from red tattoo pigment (quinacridone) with secondary spread. Contact Dermat 49(5):265–266. https://doi.org/10.1111/j.0105-1873.2003.0225h.x
Haniffa M, Ginhoux F, Wang X-N et al (2009) Differential rates of replacement of human dermal dendritic cells and macrophages during hematopoietic stem cell transplantation. J Exp Med 206(2):371–385. https://doi.org/10.1084/jem.20081633
Haniffa M, Shin A, Bigley V et al (2012) Human tissues contain CD141hi cross-presenting dendritic cells with functional homology to mouse CD103+ nonlymphoid dendritic cells. Immunity 37(1):60–73. https://doi.org/10.1016/j.immuni.2012.04.012
Hauri U (2014) Tinten für tattoos und permanent make-up/Pigmente, Konservierungsstoffe, Aromatische Amine Polyaromatische kohlenwasserstoffe und Nitrosamine. Kantonales Laboratorium, Zurich
Hauri U, Amberg J, Lüthi K-W, Baumgartner A, Gautsch S, Brüschweiler B (2009) Konformität von Tätowier- und Permanent-Make-up- Farben nicht zufriedenstellend. Bulletin des Bundesamtes für Gesundheit, Nr. 29/2009 vom 13. Juli 2009, CH-3003 Bern (Seiten 535 - 541). https://www.baselland.ch/politik-und-behorden/direktionen/volkswirtschafts-und-gesundheitsdirektion/lebensmittelsicherheit-und-veterinarwesen/Dokumente-Downloads/Kampagnenbeirchte/archiv-kampagnen/Kampagnenberichte_GG_alt/tattoo-bag.pdf/@@download/file/tattoo-bag.pdf
JRC (2015) Safety of tattoos and permanent make-up. State of play and trends in tattoo practices. Publ Office Eur Union. https://doi.org/10.2788/924128
JRC (2015) Safety of tattoos and permanent make-up: compilation of information on legislative framework and analytical methods. Publ Office Eur Union. https://doi.org/10.2788/542617
JRC (2016) Safety of tattoos and permanent make-up. Adverse health effects and experience with the Council of Europe Resolution (2008)1. Publ Office Eur Union. https://doi.org/10.2788/177900
JRC (2016) Safety of tattoos and permanent make-up: final report. EUR27947. https://doi.org/10.2788/011817
Jungmann S, Laux P, Bauer TT, Jungnickel H, Schönfeld N, Luch A (2016) From the tattoo studio to the emergency room. Dtsch Arztebl Int 113(40):672–675. https://doi.org/10.3238/arztebl.2016.0672
Kasten E (2007) Psychologische und medizinische Aspekte von Piercing, Tattoo, Selbstverletzung und anderen Körperveränderungen, vol 52. Springer, Berlin
Kluger N (2017) Viral warts and seborrhoeic keratoses on tattoos: a review of nine cases. J Eur Acad Dermatol Venereol 31(7):e340–e342. https://doi.org/10.1111/jdv.14134
Kluger N (2019) An update on cutaneous complications of permanent tattooing. Expert Rev Clin Immunol 15(11):1135–1143. https://doi.org/10.1080/1744666X.2020.1676732
Kluger N, Koljonen V (2012) Tattoos, inks, and cancer. Lancet Oncol 13(4):161–168. https://doi.org/10.1016/S1470-2045(11)70340-0
Klügl I, Hiller KA, Landthaler M, Bäumler W (2010) Incidence of health problems associated with tattooed skin: a nation-wide survey in German-speaking countries. Dermatology 221(1):43–50. https://doi.org/10.1159/000292627
Laux P, Tralau T, Tentschert J et al (2016) A medical-toxicological view of tattooing. Lancet (London, England) 387(10016):395–402. https://doi.org/10.1016/s0140-6736(15)60215-x
Lehner K, Santarelli F, Penning R et al (2011) The decrease of pigment concentration in red tattooed skin years after tattooing. J Eur Acad Dermatol Venereol 25(11):1340–1345. https://doi.org/10.1111/j.1468-3083.2011.03987.x
Lehner K, Santarelli F, Vasold R et al (2014) Black tattoos entail substantial uptake of genotoxicpolycyclic aromatic hydrocarbons (PAH) in human skin and regional lymph nodes. PLoS ONE 9(3):e92787. https://doi.org/10.1371/journal.pone.0092787
Lerche CM, Heerfordt IM, Serup J, Poulsen T, Wulf HC (2017) Red tattoos, ultraviolet radiation and skin cancer in mice. Exp Dermatol 26(11):1091–1096. https://doi.org/10.1111/exd.13383
Lerche CM, Sepehri M, Serup J, Poulsen T, Wulf HC (2015) Black tattoos protect against UVR-induced skin cancer in mice. Photodermatol Photoimmunol Photomed 31(5):261–268. https://doi.org/10.1111/phpp.12181
Mann R, Klingmüller G (1981) Electron-microscopic investigation of tattoos in rabbit skin. Arch Dermatol Res 271(4):367–372. https://doi.org/10.1007/bf00406680
McFadden JP, Basketter DA (2000) Contact allergy, irritancy and ‘danger’. Contact Dermat 42(3):123–127. https://doi.org/10.1034/j.1600-0536.2000.042003123.x
Munshi A, Parmar V, Rekhi B (2011) Post mastectomy local recurrence at the tattoo site—a proof of principle? Acta Oncol 50(4):598–599. https://doi.org/10.3109/0284186X.2010.539260
Niederer M, Hauri U, Kroll L, Hohl C (2018) Identification of organic pigments in tattoo inks and permanent make-up using laser desorption ionisation mass spectrometry [version 2; peer review: 2 approved]. F1000Research. https://doi.org/10.12688/f1000research.13035.2
Prior G (2015) Tattoo inks: legislation, pigments, metals and chemical analysis. Curr Probl Dermatol 48:152–157. https://doi.org/10.1159/000369196
RAC (2018) Opinion on an Annex XV dossier proposing restrictions on substances used in tattoo inks and permanent make-up. European Chemicals Agency, Helsinki
Regensburger J, Lehner K, Maisch T et al (2010) Tattoo inks contain polycyclic aromatic hydrocarbons that additionally generate deleterious singlet oxygen. Exp Dermatol 19(8):e275–e281. https://doi.org/10.1111/j.1600-0625.2010.01068.x
Renzoni A, Pirrera A, Novello F et al (2018) The tattooed population in Italy: a national survey on demography, characteristics and perception of health risks. Annali dell'Istituto superiore di sanita 54(2):126–136. https://doi.org/10.4415/ann_18_02_08
Sabbioni G, Hauri U (2016) Carcinogenic tattoos? Epidemiol Biostat Public Health. https://doi.org/10.2427/12018
SCCS (2012) The SCCS's notes of guidance for testing of cosmetic ingredients and their safety evaluation scientific committee on consumer safety 8th revision, SCCS/1501/12, adopted on December 2012. https://ec.europa.eu/health/sites/health/files/scientific_committees/consumer_safety/docs/sccs_s_006.pdf
Schreiver I, Hesse B, Seim C et al (2019) Distribution of nickel and chromium containing particles from tattoo needle wear in humans and its possible impact on allergic reactions. Part Fibre Toxicol 16(1):33. https://doi.org/10.1186/s12989-019-0317-1
Schreiver I, Hesse B, Seim C et al (2017) Synchrotron-based ν-XRF mapping and μ-FTIR microscopy enable to look into the fate and effects of tattoo pigments in human skin. Sci Rep 7(1):11395. https://doi.org/10.1038/s41598-017-11721-z
Schwitulla J, Brasch J, Loffler H, Schnuch A, Geier J, Uter W (2014) Skin irritability to sodium lauryl sulfate is associated with increased positive patch test reactions. Br J Dermatol 171(1):115–123. https://doi.org/10.1111/bjd.12893
Sepehri M, Hutton Carlsen K, Serup J (2016) Papulo-nodular reactions in black tattoos as markers of sarcoidosis: study of 92 tattoo reactions from a hospital material. Dermatology 232(6):679–686. https://doi.org/10.1159/000453315
Sepehri M, Sejersen T, Qvortrup K, Lerche CM, Serup J (2017) Tattoo pigments are observed in the kupffer cells of the liver indicating blood-borne distribution of tattoo ink. Dermatology 233(1):86–93. https://doi.org/10.1159/000468149
Serup J, Carlsen KH, Dommershausen N et al (2019) Identification of pigments related to allergic tattoo reactions in 104 human skin biopsies. Contact Dermat. https://doi.org/10.1111/cod.13423
Serup J, Sepehri M, Hutton Carlsen K (2016) Classification of tattoo complications in a hospital material of 493 adverse events. Dermatology 232(6):668–678. https://doi.org/10.1159/000452148
Shah H, Tiwary AK, Kumar P (2018) Transepidermal elimination: historical evolution, pathogenesis and nosology. Indian J Dermatol Venereol Leprol 84(6):753–757. https://doi.org/10.4103/ijdvl.IJDVL_396_17
Steinbrecher I, Hemmer W, Jarisch R (2004) Fallberichte. J der Deutschen Dermatologischen Gesellschaft 2(12):1007–1012. https://doi.org/10.1046/j.1439-0353.2004.04733.x
Sullivan TP, Eaglstein WH, Davis SC, Mertz P (2001) The pig as a model for human wound healing. Wound Repair Regener 9(2):66–76. https://doi.org/10.1046/j.1524-475x.2001.00066.x
Tamoutounour S, Guilliams M, Montanana Sanchis F et al (2013) Origins and functional specialization of macrophages and of conventional and monocyte-derived dendritic cells in mouse skin. Immunity 39(5):925–938. https://doi.org/10.1016/j.immuni.2013.10.004
Tang J, Xiong L, Wang S et al (2009) Distribution, translocation and accumulation of silver nanoparticles in rats. J Nanosci Nanotechnol 9(8):4924–4932. https://doi.org/10.1166/jnn.2009.1269
TätoV (2008) Verordnung über Mittel zum Tätowieren einschließlich bestimmter vergleichbarer Stoffe und Zubereitungen aus Stoffen (Tätowiermittel-Verordnung). Bundesministerium der Justiz und für Verbraucherschutz, Berlin
Tighe ME, Libby DK, Dorn SK, Hosmer JR, Peaslee GF (2017) A survey of metals found in tattoo inks. J Environ Prot 8(11):1243–1253. https://doi.org/10.4236/jep.2017.811077
van der Bent SAS, de Winter RW, Wolkerstorfer A, Rustemeyer T (2019) Red tattoo reactions, a prospective cohort on clinical aspects. J Eur Acad Dermatol Venereol 33(10):e384–e386. https://doi.org/10.1111/jdv.15677
Wenzel SM, Rittmann I, Landthaler M, Bäumler W (2013) Adverse reactions after tattooing: review of the literature and comparison to results of a survey. Dermatology 226(2):138–147. https://doi.org/10.1159/000346943
Acknowledgements
Open Access funding provided by Projekt DEAL. Aaron Katz is kindly thanked for proofreading of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they do not have potential conflicts of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Giulbudagian, M., Schreiver, I., Singh, A.V. et al. Safety of tattoos and permanent make-up: a regulatory view. Arch Toxicol 94, 357–369 (2020). https://doi.org/10.1007/s00204-020-02655-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00204-020-02655-z