Abstract
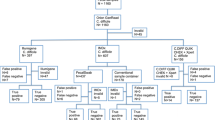
Clostridioides difficile infection is a public health problem because of it is easily spread; with harmful consequences, it is essential to reduce hospital costs and prevent its dissemination by having a precise diagnosis. The gold standard for its diagnosis is polymerase chain reaction (PCR); however, the technique is not available for all laboratories due to the high cost. New approaches using non-molecular tests to detect C. difficile and toxin A/B production has been proposed to improve cost benefits. The objective of this study is to compare molecular methods (PCR) and rapid methods (immunochromatographic test and enzymatic immunoassay). A series of tests comprising these diagnostic techniques was performed with 50 patients with a clinical diagnosis for Clostridioides difficile on GeneXpert® devices test; a calculation of the sensitivity was executed, followed by a comparison of the efficiency of all techniques. Greater sensitivity was observed in the PCR-based methods (BD MAX™ and BioFire FilmArray®) and the GDH-based assays (RIDASCREEN® and Alere Techlab®). The proposed algorithm represents minor monetary disadvantages but a significant temporal optimization of 10%. Future studies concerning both positive and negative results could be advantageous because of the possibility of calculating more method concordance indexes, such as the specificity and Kappa index, in addition to being able to indicate a monetary profit if the proposed algorithm was applied due to the nonproceeding PCR cases.

Similar content being viewed by others
References
Alcalá L et al (2008) Comparison of three commercial methods for rapid detection of clostridium difficile toxins A and B from fecal specimens. J clin Microbiol 46:3833–3835. https://doi.org/10.1128/JCM.01060-08
Alcalá L et al (2010) Comparison of ImmunoCard Toxins A&B and the new semiautomated Vidas Clostridium difficile Toxin A&B tests for diagnosis of C. difficile infection. J Clin Microbiol 48:1014–1015. https://doi.org/10.1128/JCM.01642-09
Babady NE et al (2010) Evaluation of the Cepheid Xpert Clostridium difficile Epi assay for diagnosis of Clostridium difficile infection and typing of the NAP1 strain at a cancer hospital. J Clin Microbiol 48:4519–4524. https://doi.org/10.1128/JCM.01648-10
Bartlett JG (2008) Historical perspectives on studies of Clostridium difficile and C. difficile Infection. Clin Infect Dis 46:S4–S11. https://doi.org/10.1086/521865
Burnham C-AD, Carroll KC (2013) Diagnosis of Clostridium difficile infection: an ongoing conundrum for clinicians and for clinical laboratories. Clin Microbiol Rev 26:604–630. https://doi.org/10.1128/CMR.00016-13
Buss SN et al (2015) Multicenter evaluation of the BioFire FilmArray gastrointestinal panel for etiologic diagnosis of infectious gastroenteritis. J Clin Microbiol 53:915–925. https://doi.org/10.1128/JCM.02674-14
Crobach MJT et al (2016) European society of clinical microbiology and infectious diseases: update of the diagnostic guidance document for Clostridium difficile infection. Clin Microbiol Infect 22:S63–S81. https://doi.org/10.1016/j.cmi.2016.03.010
Dalpke AH, Hofko M, Zorn M, Zimmermann S (2013) Evaluation of the fully automated BD MAX C. diff and Xpert C. difficile assays for direct detection of Clostridium difficile in stool specimens. J Clin Microbiol 51:1906–1908. https://doi.org/10.1128/JCM.00344-13
Eastwood K, Else P, Charlett A, Wilcox M (2009) Comparison of nine commercially available Clostridium difficile toxin detection assays, a real-time PCR assay for C. difficile tcdB, and a glutamate dehydrogenase detection assay to cytotoxin testing and cytotoxigenic culture methods. J Clin Microbiol 47:3211–3217. https://doi.org/10.1128/JCM.01082-09
Fenner L, Widmer AF, Goy G, Rudin S, Frei R (2008) Rapid and reliable diagnostic algorithm for detection of Clostridium difficile. J Clin Microbiol 46:328. https://doi.org/10.1128/JCM.01503-07
Gilbreath JJ, Verma P, Abbott AN, Butler-Wu SM (2014) Comparison of the Verigene Clostridium difficile, Simplexa C. difficile Universal Direct, BD MAX Cdiff, and Xpert C. difficile assays for the detection of toxigenic C. difficile. Diagn Microbiol Infect Dis 80:13–18. https://doi.org/10.1016/j.diagmicrobio.2014.06.001
Hookman P, Barkin JS (2009) Clostridium difficile associated infection, diarrhea and colitis. World J Gastroenterol 15:1554–1580. https://doi.org/10.3748/wjg.15.1554
Kachrimanidou M, Tegou Z, Chasampalioti M, Arvaniti K, Protonotariou E, Skoura L (2017) A two-step approach improves the diagnosis of Clostridium difficile infection. J Microbiol Methods 143:17–19. https://doi.org/10.1016/j.mimet.2017.09.015
Kaltsas A et al (2012) Clinical and laboratory characteristics of Clostridium difficile infection in patients with discordant diagnostic test results. J Clin Microbiol 50:1303–1307. https://doi.org/10.1128/JCM.05711-11
Kim H, Kim WH, Kim M, Jeong SH, Lee K (2014) Evaluation of a rapid membrane enzyme immunoassay for the simultaneous detection of glutamate dehydrogenase and toxin for the diagnosis of Clostridium difficile infection. Ann Lab Med 34:235–239. https://doi.org/10.3343/alm.2014.34.3.235
Lalande V, Barrault L, Wadel S, Eckert C, Petit J-C, Barbut F (2011) Evaluation of a loop-mediated isothermal amplification assay for diagnosis of Clostridium difficile infections. J Clin Microbiol 49:2714–2716. https://doi.org/10.1128/JCM.01835-10
Le Guern R, Herwegh S, Grandbastien B, Courcol R, Wallet F (2012) Evaluation of a new molecular test, the BD Max Cdiff, for detection of toxigenic Clostridium difficile in fecal samples. J Clin Microbiol 50:3089–3090. https://doi.org/10.1128/JCM.01250-12
Martínez-Meléndez A, Camacho-Ortiz A, Morfin-Otero R, Maldonado-Garza HJ, Villarreal-Treviño L, Garza-González E (2017) Current knowledge on the laboratory diagnosis of Clostridium difficile infection. World J Gastroenterol 23:1552–1567. https://doi.org/10.3748/wjg.v23.i9.1552
McElgunn CJ et al (2014) A low complexity rapid molecular method for detection of Clostridium difficile in stool. PLoS ONE 9:e83808. https://doi.org/10.1371/journal.pone.0083808
Miller S, Wiita A, Wright C, Reyes H, Liu C (2013) Evaluation of glutamate dehydrogenase immunoassay screening with toxin confirmation for the diagnosis of Clostridium difficile infection. Lab Med 44:e65–e71. https://doi.org/10.1309/LM31ZX1PFRZTGJUI
Musher DM et al (2007) Detection of Clostridium difficile toxin: comparison of enzyme immunoassay results with results obtained by cytotoxicity assay. J Clin Microbiol 45:2737–2739. https://doi.org/10.1128/JCM.00686-07
Shin B-M, Kuak EY, Lee EJ, Songer JG (2009) Algorithm combining toxin immunoassay and stool culture for diagnosis of Clostridium difficile infection. J Clin Microbiol 47:2952–2956. https://doi.org/10.1128/JCM.00609-09
Silva ROS, Vilela EG, Neves MS, Lobato FCF (2014) Evaluation of three enzyme immunoassays and a nucleic acid amplification test for the diagnosis of Clostridium difficile-associated diarrhea at a university hospital in Brazil. Rev Soc Bras Med Trop 47:447–450
Sloan LM, Duresko BJ, Gustafson DR, Rosenblatt JE (2008) Comparison of real-time PCR for detection of the tcdC gene with four toxin immunoassays and culture in diagnosis of Clostridium difficile infection. J Clin Microbiol 46:1996–2001. https://doi.org/10.1128/JCM.00032-08
Stellrecht KA et al (2014) Premarket evaluations of the IMDx C. difficile for Abbott m2000 Assay and the BD Max Cdiff assay. J Clin Microbiol 52:1423–1428. https://doi.org/10.1128/JCM.03293-13
Stockmann C et al (2015) How well does physician selection of microbiologic tests identify Clostridium difficile and other pathogens in paediatric diarrhoea? Insights using multiplex PCR-based detection. Clin Microbiol Infect 21:179.e9-179.e15. https://doi.org/10.1016/j.cmi.2014.07.011
Wei C, Wen-En L, Yang-Ming L, Shan L, Yi-Ming Z (2015) Diagnostic accuracy of loop-mediated isothermal amplification in detection of Clostridium difficile in stool samples: a meta-analysis. Archiv Med Sci AMS 11:927–936. https://doi.org/10.5114/aoms.2015.54846
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Erko Stackebrandt.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Camargo, T.S., Junior, M.S., Camargo, L.F.A. et al. Clostridioides difficile laboratory diagnostic techniques: a comparative approach of rapid and molecular methods. Arch Microbiol 203, 1683–1690 (2021). https://doi.org/10.1007/s00203-020-02148-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-020-02148-8