Abstract
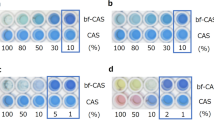
The mycolic acid layer and S-layer of Corynebacterium glutamicum have been considered as permeability barriers against lytic agents. EGTA, a calcium chelator, inhibited C. glutamicum growth at relatively lower concentrations compared with other Gram-positive bacteria. We investigated the effect of EGTA on C. glutamicum cell surface structures. Simultaneous addition of EGTA and lysozyme resulted in cell lysis, whereas addition of these reagents separately had no such effect. Analysis of cell surface proteins showed that CspB, an S-layer protein, was released into the culture media and degraded to several sizes upon EGTA treatment. These findings suggest that EGTA treatment causes release and proteolysis of the CspB protein, resulting in increased cell surface permeability. FE-SEM visualization further confirmed alteration of cell surface structures in EGTA-treated cells. This is the first report suggesting the importance of calcium ions in cell surface integrity of C. glutamicum.





Similar content being viewed by others
References
Avila-Calderón ED, Araiza-Villanueva MG, Cancino-Diaz JC, López-Villegas EO, Sriranganathan N, Boyle SM, Contreras-Rodríguez A (2015) Roles of bacterial membrane vesicles. Arch Microbiol 197:1–10. doi:10.1007/s00203-014-1042-7
Baranova E, Fronzes R, Garcia-Pino A, Van Gerven N, Papapostolou D, Péhau-Arnaudet G, Pardon E, Steyaert J, Howorka S, Remaut H (2012) SbsB structure and lattice reconstruction unveil Ca2+ triggered S-layer assembly. Nature 487:119–122. doi:10.1038/nature11155
Bayan N, Houssin C, Chami M, Leblon G (2003) Mycomembrane and S-layer: two important structures of Corynebacterium glutamicum cell envelope with promising biotechnology applications. J Biotechnol 104:55–67. doi:10.1016/S0168-1656(03)00163-9
Becker J, Wittmann C (2012) Bio-based production of chemicals, materials and fuels -Corynebacterium glutamicum as versatile cell factory. Curr Opin Biotechnol 23:631–640. doi:10.1016/j.copbio.2011.11.012
Billman-Jacobe H, Wang L, Kortt A, Stewart D, Radford A (1995) Expression and secretion of heterologous proteases by Corynebacterium glutamicum. Appl Environ Microbiol 61:1610–1613
Chami M, Bayan N, Peyret JL, Gulik-Krzywicki T, Leblon G, Shechter E (1997) The S-layer protein of Corynebacterium glutamicum is anchored to the cell wall by its C-terminal hydrophobic domain. Mol Microbiol 23:483–492. doi:10.1046/j.1365-2958.1997.d01-1868.x
Clapham DE (2007) Calcium signaling. Cell 131:1047–1058. doi:10.1016/j.cell.2007.11.028
Daniel RA, Errington J (2003) Control of cell morphogenesis in bacteria: two distinct ways to make a rod-shaped cell. Cell 113:767–776. doi:10.1016/S0092-8674(03)00421-5
Date M, Itaya H, Matsui H, Kikuchi Y (2006) Secretion of human epidermal growth factor by Corynebacterium glutamicum. Lett Appl Microbiol 42:66–70. doi:10.1111/j.1472-765X.2005.01802.x
Domínguez DC, Guragain M, Patrauchan M (2015) Calcium binding proteins and calcium signaling in prokaryotes. Cell Calcium 57:151–165. doi:10.1016/j.ceca.2014.12.006
Freudl R (2017) Beyond amino acids: Use of the Corynebacterium glutamicum cell factory for the secretion of heterologous proteins. J Biotechnol. doi:10.1016/j.jbiotec.2017.02.023
Gebhardt H, Meniche X, Tropis M, Krämer R, Daffé M, Morbach S (2007) The key role of the mycolic acid content in the functionality of the cell wall permeability barrier in Corynebacterineae. Microbiology 153:1424–1434. doi:10.1099/mic.0.2006/003541-0
Hansmeier N, Bartels FW, Ros R, Anselmetti D, Tauch A, Pühler A, Kalinowski J (2004) Classification of hyper-variable Corynebacterium glutamicum surface-layer proteins by sequence analysis and atomic force microscopy. J Biotechnol 112:177–193. doi:10.1016/j.jbiotec.2004.03.020
Hermann T (2003) Industrial production of amino acids by coryneform bacteria. J Biotechnol 104:155–172. doi:10.1016/S0168-1656(03)00149-4
Hermann T, Finkemeier M, Pfefferle W, Wersch G, Krämer R, Burkovski A (2000) Two-dimensional electrophoretic analysis of Corynebacterium glutamicum membrane fraction and surface proteins. Electrophoresis 21:654–659. doi:10.1002/(SICI)1522-2683(20000201)21:3<654::AID-ELPS654>3.0.CO;2-1
Hermann T, Pfefferie W, Baumann C, Busker E, Schaffer S, Bott M, Sahm H, Dusch N, Kalinowski J, Pühler A, Bendt AK, Krämer R, Burkovski A (2001) Proteome analysis of Corynebacterium glutamicum. Electrophoresis 22:1712–1723. doi:10.1002/1522-2683(200105)22:9<1712::AID-ELPS1712>3.0.CO;2-G
Hirasawa T, Wachi M (2017) Glutamate fermentation-2: Mechanism of l-glutamate overproduction in Corynebacterium glutamicum. Adv Biochem Eng Biotechnol 159:57–72. doi:10.1007/10_2016_26
Hirasawa T, Wachi M, Nagai K (2000) A mutation in the Corynebacterium glutamicum ltsA gene causes susceptibility to lysozyme, temperature-sensitive growth, and l-glutamate production. J Bacteriol 182:2696–2701. doi:10.1128/JB.182.10.2696-2701.2000
Hirasawa T, Wachi M, Nagai K (2001) l-glutamate production by lysozyme-sensitive Corynebacterium glutamicum ltsA mutant strains. BMC Biotechnol 1:9. doi:10.1186/1472-6750-1-9
Jaiswal R, Panda D (2009) Different assembly properties of Escherichia coli and Mycobacterium tuberculosis FtsZ: an analysis using divalent calcium. J Biochem 146:733–742. doi:10.1093/jb/mvp120
Joliff G, Mathieu L, Hahn V, Bayan N, Duchiron F, Renaud M, Shechter E, Leblon G (1992) Cloning and nucleotide sequence of the csp1 gene encoding PS1, one of the two major secreted proteins of Corynebacterium glutamicum: the deduced N-terminal region of PS1 is similar to the Mycobacterium antigen 85 complex. Mol Microbiol 6:2349–2362. doi:10.1111/j.1365-2958.1992.tb01410.x
Kikuchi Y, Date M, Yokoyama K, Umezawa Y, Matsui H (2003) Secretion of active-form Streptoverticillium mobaraense transglutaminase by Corynebacterium glutamicum: processing of the pro-transglutaminase by a cosecreted subtilisin-Like protease from Streptomyces albogriseolus. Appl Environ Microbiol 69:358–366. doi:10.1128/AEM.69.1.358-366.2003
Kikuchi Y, Date M, Itaya H, Matsui K, Wu LF (2006) Functional analysis of the twin-arginine translocation pathway in Corynebacterium glutamicum ATCC 13869. Appl Environ Microbiol 72:7183–7192. doi:10.1128/AEM.01528-06
Kinoshita S, Udaka S, Shimono M (1957) Studies of the amino acid fermentation: production of l-glutamic acid by various microorganisms. J Gen Appl Microbiol 3:193–205. doi:10.2323/jgam.3.193
Letek M, Ordóñez E, Vaquera J, Margolin W, Flärdh K, Mateos ML, Gil AJ (2008) DivIVA is required for polar growth in the MreB-lacking rod-shaped actinomycete Corynebacterium glutamicum. J Bacteriol 190:3283–3292. doi:10.1128/JB.01934-07
Machaca K (2010) Ca2+ signaling, genes and the cell cycle. Cell Calcium 48:243–250. doi:10.1016/j.ceca.2010.10.003
Marienfeld S, Uhlemann EM, Schmid R, Krämer R, Burkovski A (1997) Ultrastructure of the Corynebacterium glutamicum cell wall. Antonie Van Leeuwenhoek 72:291–297. doi:10.1023/A:1000578811089
Matsuda Y, Itaya H, Kitahara Y, Theresia NM, Kutukova EA, Yomantas YAV, Date M, Kikuchi Y, Wachi M (2014) Double mutation of cell wall proteins CspB and PBP1a increases secretion of the antibody Fab fragment from Corynebacterium glutamicum. Microb Cell Fact 13:1–10. doi:10.1186/1475-2859-13-56
McBroom AJ, Kuehn MJ (2007) Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol Microbiol 63:545–558. doi:10.1111/j.1365-2958.2006.05522.x
Ochiai K, Takayama K, Kawamoto I (1987) Cytological changes in Corynebacterium glutamicum cell envelope structure caused by biotin deficiency. Actinomycetologica 1:31–42. doi:10.3209/saj.1_31
Ojima Y, Mohanadas T, Kitamura K, Nunogami S, Yajima R, Taya M (2017) Deletion of degQ gene enhances outer membrane vesicle production of Shewanella oneidensis cells. Arch Microbiol 199:415–423. doi:10.1007/s00203-016-1315-4
Payret JL, Bayan N, Joliff G, Gulik-Krzywicki T, Mathieu L, Schechter E, Leblon G (1993) Characterization of the cspB gene encoding PS2, an ordered surface-layer protein in Corynebacterium glutamicum. Mol Microbiol 9:97–109. doi:10.1111/j.1365-2958.1993.tb01672.x
Portevin D, De Sousa-D’Auria C, Houssin C, Grimaldi C, Chami M, Daffe M, Gullhot C (2004) A polyketide synthase catalyzes the last condensation step of mycolic acid biosynthesis in mycobacteria and related organisms. Proc Natl Acad Sci USA 101:314–319. doi:10.1073/pnas.0305439101
Prados-Rosales R, Baena A, Martinez LR, Luque-Garcia J, Kalscheuer R, Veeraraghavan U, Camara C, Nosanchuk JD, Besra GS, Chen B, Jimenez J, Glatman-Freedman A, Jacobs WR Jr, Porcelli SA, Casadevall A (2011) Mycobacteria release active membrane vesicles that modulate immune responses in a TLR2-dependent manner in mice. J Clin Invest 121:1471–1483. doi:10.1172/JCI44261
Prados-Rosales R, Weinrick BC, Piqué DG, Jacobs WR Jr, Casadevall A, Rodriguez GM (2014) Role for Mycobacterium tuberculosis membrane vesicles in iron acquisition. J Bacteriol 196(6):1250–1256. doi:10.1128/JB.01090-13
Puech V, Chami M, Lemassu A, Lanéelle MA, Schiffler B, Gounon P, Bayan N, Benz R, Daffé M (2001) Structure of the cell envelope of corynebacteria: importance of the non-covalently bound lipids in the formation of the cell wall permeability barrier and fracture plane. Microbiology 147:1365–1382. doi:10.1099/00221287-147-5-1365
Sahm H, Eggeling L, Eikmanns B, Krämer R (1996) Construction of l-lysine-, l-threonine-, and l-isoleucine-overproducing strains of Corynebacterium glutamicum. Ann N Y Acad Sci 782:25–39. doi:10.1111/j.1749-6632.1996.tb40544.x
Sála M, Sleytr UB (2000) S-Layer proteins. J Bacteriol 182:859–868. doi:10.1128/JB.182.4.859-868.2000
Suzuki N, Watanabe K, Okibe N, Tsuchida Y, Inui M, Yukawa H (2009) Identification of new secreted proteins and secretion of heterologous amylase by C. glutamicum. Appl Microbiol Biotechnol 82:491–500. doi:10.1007/s00253-008-1786-6
Teramoto H, Watanabe K, Suzuki N, Inui M, Yukawa H (2011) High yield secretion of heterologous proteins in Corynebacterium glutamicum using its own Tat-type signal sequence. Appl Microbiol Biotechnol 91:677–687. doi:10.1007/s00253-011-3281-8
Udaka S (1960) Screening method for microorganisms accumulating metabolites and its use in the isolation of Micrococcus glutamicus. J Bacteriol 79:754–755
Vertès AA (2013) Protein secretion systems of Corynebacterium glutamicum. In: Yukawa H, Inui M (eds) Corynebacterium glutamicum biology and biotechnology. Springer, Heidelberg, pp 351–379
Wachi M, Iwai N, Kunihasa A, Nagai K (1999) Irregular nuclear localization and anucleate cell production in Escherichia coli induced by Ca2+ chelator, EGTA. Biochemie 81:909–913. doi:10.1016/S0300-9084(99)00204-7
Walker SG, Karunaratne N, Ravenscroft N, Smit J (1994) Characterization of mutants of Caulobacter crescentus defective in surface attachment of the paracrystalline surface layer. J Bacteriol 176:6312–6323
Yang J, Yang S (2017) Comparative analysis of Corynebacterium glutamicum genomes: a new perspective for the industrial production of amino acids. BMC Genomics 18(Suppl 1):940. doi:10.1186/s12864-016-3255-4
Yu XC, Margolin W (1997) Ca2+-mediated GTP-dependent dynamic assembly of bacterial cell division protein FtsZ into asters and polymer networks in vitro. EMBO J 16:5455–5463. doi:10.1093/emboj/16.17.5455
Yukawa H, Inui M, Vertès AA (2007a) Genomes and genome-level engineering of amino-acid producing bacteria. In: Wendisch VF (ed) Amino acid biosynthesis—pathways, regulation and metabolic engineering, microbiology monographs. Springer, Heidelberg, pp 349–401
Yukawa H, Omumasaba CA, Nonaka H, Kos P, Okai N, Suzuki N, Suda M, Tsuge Y, Watanabe J, Ikeda Y, Vertes AA, Inui M (2007b) Comparative analysis of the Corynebacterium glutamicum group and complete genome sequence of strain R. Microbiology 153:1042–1058. doi:10.1099/mic.0.2006/003657-0
Acknowledgements
This work was supported in part by a Grant-in-Aid for challenging Exploratory Research (25660088, to M. W.) from the Japan Society for Promotion of Science. The authors thank the members of the Suzukakedai Material Analysis Division, Technical Department, Tokyo Institute of Technology for FE-SEM analysis.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by Jorge Membrillo-Hernández.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Theresia, N.M., Aida, K., Takada, A. et al. Effects of EGTA on cell surface structures of Corynebacterium glutamicum . Arch Microbiol 200, 281–289 (2018). https://doi.org/10.1007/s00203-017-1445-3
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-017-1445-3