Abstract
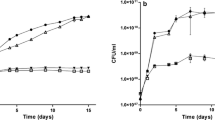
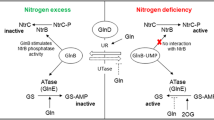
Nitrogen fixation in Azospirillum brasilense is regulated at transcriptional and post-translational levels. Post-translational control occurs through the reversible ADP-ribosylation of dinitrogenase reductase (Fe Protein), mediated by the dinitrogenase reductase ADP-ribosyltransferase (DraT) and dinitrogenase reductase glycohydrolase (DraG). Although the DraT and DraG activities are regulated in vivo, the molecules responsible for such regulation remain unknown. We have constructed broad-host-range plasmids capable of over-expressing, upon IPTG induction, the regulatory enzymes DraT and DraG as six-histidine-N-terminal fused proteins (His). Both DraT-His and DraG-His are functional in vivo. We have analyzed the effects of DraT-His and DraG-His over-expression on the post-translational modification of Fe Protein. The DraT-His over-expression led to Fe Protein modification in the absence of ammonium addition, while cells over-expressing DraG-His showed only partial ADP-ribosylation of Fe Protein by adding ammonium. These results suggest that both DraT-His and DraG-His lose their regulation upon over-expression, possible by titrating out negative regulators.





Similar content being viewed by others
References
Bashan Y, Holguin G (1997) Azospirillum—plant relationships: environmental and physiological advances (1990–1996). Can J Microbiol 43:103–121
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254
Burnette WN (1981) “Western Blotting”: electrophoretic transfer of proteins from sodium dodecyl sulfate-polyacrylamide gels to unmodified nitrocellulose and radiographic detection with antibody and radioiodinated protein A. Anal Biochem 112:195–203
Burris RH (1991) Nitrogenases. J Biol Chem 266:9339–9342
Drepper T, Gross S, Yakunin AF, Hallenbeck PC, Masepohl B, Klipp W (2003) Role of GlnB and GlnK in ammonium control of both nitrogenase systems in the phototrophic bacterium Rhodobacter capsulatus. Microbiology 149:2203–2212
Grunwald SK, Lies DP, Roberts GP, Ludden PW (1995) Post-translational regulation of nitrogenase in Rhodospirillum rubrum strains overexpressing the regulatory enzymes dinitrogenase reductase ADP-ribosyltransferase and dinitrogenase reductase activating glycohydrolase. J Bacteriol 177:628–635
Halbleid CM, Ludden PW (1999) Characterization of the interaction of dinitrogenase reductase-activating glycohydrolase from Rhodospirillum rubrum with bacterial membranes. Arch Microbiol 172:51–58
Halbleid CM, Zhang Y, Ludden PW (2000) Regulation of dinitrogenase reductase ADP-ribosyltransferase and dinitrogenase reductase-activating glycohydrolase by a redox-dependent conformational change of nitrogenase Fe protein. J Biol Chem 275:3493–3500
Harlow E, Lane D (1988) Antibody, a laboratory manual, 3rd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
HartmannA,Fu H, Burris RH (1986) Regulation of nitrogenase activity by ammonium chloride in Azospirillum spp. J Bacteriol 165:864–870
Kim K, Zhang Y, Roberts GP (2004) Characterization of altered regulation variants of dinitrogenase reductase-activating glycohydrolase from Rhodospirillum rubrum. FEBS Lett 559:84–88
Klassen G, Souza EM, Yates MG, Rigo LU, Inaba J, Pedrosa FO (2001) Control of nitrogenase reactivation by the GlnZ protein in Azospirillum brasilense. J Bacteriol 183:6710–6713
Kleiner D, Paul W, Merrick MJ (1988) Construction of multicopy expression vectors for regulated over-production of proteins in Klebsiella pneumoniae and other enteric bacteria. J Gen Microbiol 134:1779–1784
Laemmli UK (1970) Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 277:680–685
Ljungstrom E, Yates MG, Nordlund S (1989) Purification of the activating enzyme for the Fe-Protein of nitrogenase from Azospirillum brasilense. Biochim Biophys Acta 994:210–214
Lowery RG, Ludden PW (1988) Purification and properties of dinitrogenase reductase ADP-ribosyltransferase from the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem 263:16714–16719
Machado HB, Funayama S, Rigo LU, Pedrosa FO (1991) Excretion of ammonium by Azospirillum brasilense mutants resistant to ethylenediamine. Can J Microbiol 37:549–553
Martin DE, Reinhold-Hurek B (2002) Distinct roles of PII-like signal transmitter proteins and amtB in regulation of nif gene expression, nitrogenase activity, and posttranslational modification of NifH in Azoarcus sp. strain BH72. J Bacteriol 184:2251–2259
Nordlund S, Eriksson U, Baltscheffsky H (1977) Necessity of a membrane component for nitrogenase activity in Rhodospirillum rubrum. Biochim Biophys Acta 462:187–195
Pawlowski A, Riedel KU, Klipp W, Dreiskemper P, Gross S, Bierhoff H, Drepper T, Masepohl B (2003) Yeast two-hybrid studies on interaction of proteins involved in regulation of nitrogen fixation in the phototrophic bacterium Rhodobacter capsulatus. J Bacteriol 185:5240–5247
Pedrosa FO, Yates MG (1984) Regulation of nitrogen fixation (nif) genes of Azospirillum brasilense by nifA and ntrC (glnG) type genes. FEMS Microbiol Lett 55:95–101
Saari LL, Triplett EW, Ludden PW (1984) Purification and properties of the activating enzyme for iron protein of nitrogenase from the photosynthetic bacterium Rhodospirillum rubrum. J Biol Chem 259:15502–15508
Sambrook J, Fritsch EF, Maniatis T (1989) Molecular cloning a laboratory manual, 2nd edn. Cold Spring Harbor Laboratory Press, Cold Spring Harbor
Simon R, Priefer U, Puhler A (1983) A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram-negative bacteria. Biotechnology 1:784–791
Spaink HP, Okker RJH, Wijffelman CA, Pees E, Lugtenberg BJJ (1987) Promoters in the nodulation region of the Rhizobium leguminosarum Sym plasmid pRLIJI. Plant Mol Biol 9:27–39
Steenhout O, Vanderleyden J (2000) Azospirillum, a free-living nitrogen-fixing bacterium closely associated with grasses: genetic, biochemical and ecological aspects. FEMS Microbiol Rev 24:487–506
Yakunin AF, Hallenbeck PC (2002) AmtB is necessary for NH +4 induced nitrogenase switch-off and ADP-ribosylation in Rhodobacter capsulatus. J Bacteriol 184:4081–4088
Zhang Y, Burris RH, Roberts GP (1992) Cloning, sequencing, mutagenesis, and functional characterization of draT and draG genes from Azospirillum brasilense. J Bacteriol 174:3364–3369
Zhang Y, Burris RH, Ludden PW, Roberts GP (1993) Post-translational regulation of nitrogenase activity by anaerobiosis and ammonium in Azospirillum brasilense. J Bacteriol 175:6781–6788
Zhang Y, Burris RH, Ludden PW, Roberts GP (1997) Regulation of nitrogen fixation in Azospirillum brasilense. FEMS Microbiol Lett 152:195–204
Zhang Y, Pohlmann EL, Halbleib CM, Ludden PW, Roberts GP (2001a) Effect of PII and its homolog GlnK on reversible ADP-ribosylation of dinitrogenase reductase by heterologous expression of the Rhodospirillum rubrum dinitrogenase reductase ADP-ribosyl transferase-dinitrogenase reductase activating glycohydrolase regulatory system in Klebsiella pneumoniae. J Bacteriol 183:1610–1620
Zhang Y, Pohlmann EL, Ludden PW, Roberts GP (2001b) Functional characterization of three GlnB homologs in the photosynthetic bacterium Rhodospirillum rubrum: roles in sensing ammonium and energy status. J Bacteriol 183:6159–6168
Acknowledgements
We are grateful to Yaoping Zhang, Gary Roberts, and Paul Ludden for providing the A. brasilense UB2 and UB4 strains. We thank Cândido J.T. Pereira and Antônio L. Andrade for assistance in antibody production. We also thank Valter A. de Baura, Roseli Prado, and Julieta Pie for technical assistance. This work was supported by PRONEX, CNPq, CAPES, Fundação Araucária and Paraná Tecnologia.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Huergo, L.F., Souza, E.M., Steffens, M.B.R. et al. Effects of over-expression of the regulatory enzymes DraT and DraG on the ammonium-dependent post-translational regulation of nitrogenase reductase in Azospirillum brasilense. Arch Microbiol 183, 209–217 (2005). https://doi.org/10.1007/s00203-005-0763-z
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00203-005-0763-z