Abstract
Summary
The effect of romosozumab is affected by previous osteoporosis treatment. Here we showed that the duration of the previous treatment just before romosozumab affects the therapeutic effect of romosozumab. Using denosumab and oral bisphosphonates for more than 1 year attenuates the effect of romosozumab.
Introduction
As an anti-sclerostin antibody, romosozumab suppresses bone resorption and stimulates bone formation. We investigated whether the effectiveness of 12 months of romosozumab treatment depended on the duration of previous treatment with teriparatide, denosumab, or oral bisphosphonates.
Methods
In total, 259 osteoporosis patients received subcutaneous injections of romosozumab (210 mg) every 4 weeks during 2019 and 2020. This study was designed as a pre–post comparison. The end points were the percent changes of bone mineral density (BMD) after 12 months of romosozumab treatment. The patients were divided into seven groups depending on the type and duration of previous treatment before starting romosozumab as follows: non-previous treatment group, change from teriparatide used for 1 year or less/more than 1 year, change from denosumab used for 1 year or less/more than 1 year, and change from oral bisphosphonates used for 1 year or less/more than 1 year.
Results
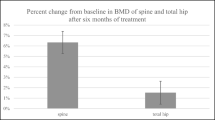
The effects of previous treatment with teriparatide on the effectiveness of 12-month romosozumab did not clearly depend on the duration of treatment (p > 0.05). In contrast, the effects of previous treatments with denosumab or oral bisphosphonates on the effectiveness of 12-month romosozumab depended on the previous treatment duration, which was reflected by the differences in percent change of the spine BMD (both p < 0.05), however, there were no significant differences in the percent change of the total hip BMD (both p > 0.05).
Conclusion
The duration of the previous treatment affected the effectiveness of romosozumab. Using denosumab and oral bisphosphonate for more than 1 year attenuated the effect of romosozumab.




Similar content being viewed by others
Data availability
The data are available from the corresponding authors upon reasonable request.
Code availability
Not applicable.
References
Genant HK, Cooper C, Poor G et al (1999) Interim report and recommendations of the World Health Organization taskforce for osteoporosis. Osteoporos Int 10:259–264. https://doi.org/10.1007/s001980050224
Cosman F, Beur SJ, LeBoff MS et al (2014) National Osteoporosis Foundation (2013) Clinician’s guide to prevention and treatment of osteoporosis. Osteoporosis Int 25:2359–2381. https://doi.org/10.1007/s00198-014-2794-2
National Osteoporosis Foundation (2002) America’s bone health: the state of osteoporosis and low bone mass in our nation. National Osteoporosis Foundation, Washington (DC)
International Osteoporosis Foundation (2020) The facts about osteoporosis and its impact. http://www.osteofound.org/press_centre/fact_sheet.html. Accessed 3 May 2021
Lewiecki EM, Wright NC, Curtis JR et al (2018) Hip fracture trends in the United States, 2002 to 2015. Osteoporos Int 29:717–722. https://doi.org/10.1007/s00198-017-4345-0
Ebina K, Hirao M, Tsuboi H et al (2020) Effects of prior osteoporosis treatment on early treatment response of romosozumab in patients with postmenopausal osteoporosis. Bone 140:115574. https://doi.org/10.1016/j.bone.2020.115574
Tominaga A, Wada K, Okazaki K et al (2021) Early clinical effects, safety, and predictors of the effects of romosozumab treatment in osteoporosis patients: one-year study. Osteoporosis Int 1–11. https://doi.org/10.1007/s00198.021.05925.3
Cosman F, Crittenden DB, Adachi JD et al (2016) Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 375:1532–1543. https://doi.org/10.1056/NEJMoa1607948
Baron R, Rawadi G (2007) Targeting the Wnt/beta-catenin pathway to regulate bone formation in the adult skeleton. Endocrinology 148(6):2635–2643. https://doi.org/10.1210/en.2007-0270
Ominsky MS, Vlasseros F, Jolette J et al (2010) Two doses of sclerostin antibody in cynomolgus monkeys increases bone formation, bone mineral density, and bone strength. J Bone Miner Res 25:948–959. https://doi.org/10.1002/jbmr.14
Li X, Warmington KS, Niu QT et al (2011) Inhibition of sclerostin by monoclonal antibody increases bone formation, bone mass, and bone strength in aged male rats. J Bone Miner Res 25:2647–2656. https://doi.org/10.1002/jbmr.182
Bandeira L, Lewiecki EM, Bilezikian JP (2017) Romosozumab for the treatment of osteoporosis. Expert Opin Biol Ther 17:255–263. https://doi.org/10.1080/14712598.2017.1280455
Igarashi Y, Lee MY, Matsuzaki S (2002) Acid phosphatase as markers of bone metabolism. J Chromatogr B 781:345–358. https://doi.org/10.1016/s1570-0232(02)00431-2
Eastell R, Krege JH, Chen P, Glass EV, Reginster JY (2006) Development of analgorism for using P1NP to monitor treatment of patients with teriparatide. Curr Med Res Opin 22:61–66. https://doi.org/10.1185/030079905X75096
Fogelman I, Blake GM (2000) Different approaches to bone densitometry. J Nucl Med 41:2015–2025
Ominsky MS, Brown DL, Van G et al (2015) Differential temporal effects of sclerostin antibody and parathyroid hormone on cancellous and cortical bone and quantitative differences in effects on the osteoblast lineage in young intact rats. Bone 81:380–391. https://doi.org/10.1016/j.bone.2015.08.007
Shimizu T, Atira K, Murota E et al (2021) Effects after starting or switching from bisphosphonate to romosozumab or denosumab in Japanese postmenopausal patients. J Bone Miner Metab 13:1–8. https://doi.org/10.1007/s00774-021-01226-1
Popp AW, Varathan N, Buffat H et al (2018) Bone mineral density changes after 1 year of denosumab discontinuation in postmenopausal women with long-term denosumab treatment for osteoporosis. Calcif Tissue Int 103:50–54. https://doi.org/10.1007/s00223.018.0394.4
Solling ASK, Harslof T, Kaal A, Rejnmark L, Langdahl B (2016) Hypercalcemia after discontinuation of long-term denosumab treatment. Osteoporos Int 27:2383–2386. https://doi.org/10.1007/s00198.016.3535.5
McClung MR, Lewiecki EM, Geller ML et al (2013) Effect of denosumab on bone mineral density and biochemical markers of bone turnover: 8-year results of a phase 2 clinical trial. Osteoporos Int 24:227–235. https://doi.org/10.1007/s00198-012-2052-4
Bone HG, Chapurlat R, Brandi M-L et al (2013) The effect of three or six years of denosumab exposure in women with postmenopausal osteoporosis: results from the freedom extension. J Clin Endocrinol Metab 98:4483–4492. https://doi.org/10.1210/jc.2013-1597
Takada J, Dinavahi R, Miyauchi A et al (2020) Relationship between P1NP, a biochemical marker of bone turnover, and bone mineral density in patients transitioned from alendronate to romosozumab or teriparatide: a post hoc analysis of the STRUCTURE trial. J Bone Miner Metab 38:310–315. https://doi.org/10.1007/s00774.019.01057.1
Bone HG, Hosking D, Devogelaer JP et al (2004) Ten years’ experience with alendronate for osteoporosis in postmenopausal women. N Engl J Med 350:1189–1199
Bauer DC, Garnero P, Hochberg MC et al (2006) Pre-treatment bone turnover and fracture efficacy of alendronate: the fracture intervention trial. J Bone Miner Res 21:292–299. https://doi.org/10.1359/JBMR.051018
Fogelman I, Ribot C, Smith R et al (2000) Risedronate reverse bone loss in postmenopausal women with low bone mass: results from a multinational, double-blind, placebo-controlled trail. J Clin Endocrinol Metab 85:1895–1900. https://doi.org/10.1210/jcem.85.5.6603
Acknowledgements
The authors would like to thank Enago (www.enago.com) for the manuscript review and editing support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethics approval
All procedures performed in this study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. The study was approved by the Tokyo Women’s Medical University Ethics Committee, number 5596.
Consent to participate
Informed consent was obtained from all participants included in the study.
Consent for publication
Not applicable.
Conflict of interest
Ayako Tominaga, Ken Okazaki, Hideharu Nishi, Yasushi Terayama, Yasuteru Kodama, and Yoshiharu Kato declare that they have no conflicts of interest. Keiji Wada received a speaking fee from Amgen Inc.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Tominaga, A., Wada, K., Okazaki, K. et al. Effect of the duration of previous osteoporosis treatment on the effect of romosozumab treatment. Osteoporos Int 33, 1265–1273 (2022). https://doi.org/10.1007/s00198-021-06261-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00198-021-06261-2